This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Development of a novel bispecific antibody therapy to overcome myeloma heterogeneity
Multiple myeloma is still an incurable hematological malignancy. One of the reasons is that myeloma cells can be heterogenous and acquire resistance after anti-myeloma treatment. Immunotherapy is an attractive strategy to target myeloma cells with drug resistance. A next-generation modality that can safely and effectively strengthen immunotherapeutic effects while overcoming the characteristics of myeloma cells is needed in order to break through these obstacles.
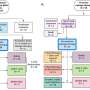
In a new study published in the Blood Journal, researchers have developed a new modality, referred to as Bridging-Bispecific T-cell Engager (B-BiTE). B-BiTE was able to bind to both to the Fc region of human immunoglobulin G monoclonal antibody (mAb) and human CD3 molecule expressed by T cells.
When B-BiTE was applied to Daratumumab (Dar) specific for CD38 and Elotuzumab (Elo) reactive for SLAMF7, which are major clinical mAbs for the treatment of myeloma, human T cells as well as NK cells successfully and safely activated for a panel of different myeloma cells in the presence of Dar/B-BiTE or Elo/B-BiTE, resulting in dual-lymphoid activation.
In addition, anti-myeloma effects mediated by an mAb/B-BiTE complex were enhanced as compared to those in the presence of mAb alone. Importantly, using an in vivo myeloma model, researchers have shown that sequential immunotherapy using two different B-BiTE-based bispecifics, Dar/B-BiTE followed by Elo/B-BiTE, appeared to circumvent antigen escape by myeloma cells and sufficiently induced deep and durable anti-myeloma responses relative to those induced by mAb/B-BiTE monotherapy or sequential therapy with two mAbs without B-BiTE.
Based on these findings, clinically available antibodies armed with B-BiTE have the potential to augment anti-myeloma effects. This approach would facilitate the easy and rapid preparation of B-BiTE-based bispecifics using a variety of clinical mAbs for the treatment of not only myeloma but also other refractory malignancies.
More information: Tatsuya Konishi et al, Reinforced anti-myeloma therapy via dual-lymphoid activation mediated by a panel of antibodies armed with Bridging-BiTE, Blood Journal (2023). DOI: 10.1182/blood.2022019082