This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Trial shows a single dose of an experimental therapy reduces lipoprotein(a) by more than 94% for nearly a year

Findings from a Phase I trial reported by a Cleveland Clinic physician show that a single dose of an experimental therapy produced greater than 94% reductions in blood levels of lipoprotein(a), a key driver of heart disease risk, with the results lasting for nearly a year.
Results from the "Efficacy and Safety of Lepodisiran: An Extended Duration Short-Interfering RNA Targeting Lipoprotein(a) Study" were presented during a late-breaking science session at the American Heart Association's Scientific Sessions 2023 and simultaneously published in the Journal of the American Medical Association (JAMA).
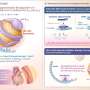
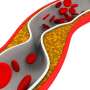
Lipoprotein(a), often shortened to just Lp(a), is made in the liver and has similarities to LDL, also known as low-density lipoprotein or "bad cholesterol." Unlike other types of cholesterol particles, Lp(a) levels are 80–90% genetically determined. The structure of the Lp(a) particle causes the accumulation of plaque in arteries which greatly increases the risk of heart attacks and strokes.
Although effective therapies exist to reduce the risk of heart disease by lowering LDL cholesterol and other lipids, currently there are no approved drug treatments to lower Lp(a). Since Lp(a) levels are determined by a person's genes, lifestyle changes (diet or exercise) have no effect.
In the trial, participants who received an injection of lepodisiran had lipoprotein(a) levels reduced by the top dose as much as 96% within two weeks and maintained levels more than 94% below baseline for 48 weeks. The drug is a small interfering RNA (siRNA) therapeutic that blocks the messenger RNA needed to manufacture a key component of lipoprotein(a) in the liver.
The findings add lepodisiran to the growing list of therapies that could be promising treatments for atherosclerotic cardiovascular diseases in people with high levels of Lp(a), which is estimated to affect 64 million people in the United States and 1.4 billion people worldwide.
"These results showed that this therapy was well tolerated and produced very long-duration reductions in Lp(a), an important risk factor that leads to heart attack, stroke and aortic stenosis," said lead author Steven Nissen, M.D., Chief Academic Officer of the Heart, Vascular & Thoracic Institute at Cleveland Clinic.
In the trial, researchers enrolled 48 patients in the U.S. and Singapore with a mean age of 47. Investigators studied six different dosages and a placebo, which were all administered as injections. Participants were monitored for up to 48 weeks after administration.
Maximum Lp(a) plasma concentrations were reduced by 49% from baseline levels for the 4 mg dose and up to 96% for the 608 mg dose vs. a 5% decrease for the placebo. No safety issues were observed, and the only tolerability issue was mild injection site reactions.
"Despite the strong evidence of the importance of elevated Lp(a) as a risk factor for heart disease, effective treatment has been elusive," said Dr. Nissen. "This approach to treatment gives hope to the 20% of the world's population who have elevated Lp(a) levels."
A Phase II trial studying lepodisiran is currently underway. Eli Lilly and Company (Lilly) is developing lepodisiran.
More information: Steven E. Nissen et al, Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a), JAMA (2023). DOI: 10.1001/jama.2023.21835