This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Discovery of drug candidate to potentially tackle ER-positive breast cancer
An international team of researchers, led by Pfizer in collaboration with Monash University and the Australian-based Cancer Therapeutics Cooperative Research Center, have discovered a pre-clinical drug candidate demonstrating anti-tumor activity in Estrogen Receptor (ER) positive breast cancer models.
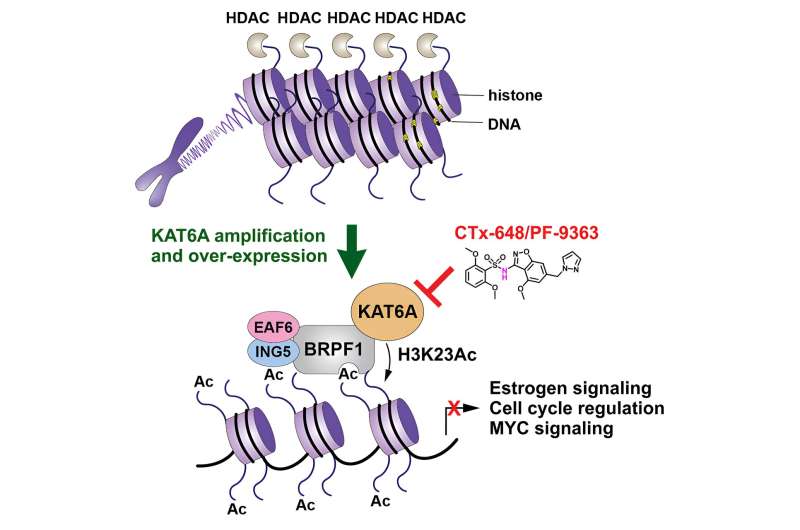
The study, published in Cell Chemical Biology, describes the identification of a highly potent, selective and orally bioavailable "KAT6A/B" inhibitor called "CTx-648," which led to promising tumor growth inhibition in ER-positive breast cancer models in mice.
The team of researchers also found CTx-648 treatment led to anti-tumor activity in tumors resistant to hormone therapy, a common line of treatment for ER-positive breast cancer patients. As such, the discovery of CTx-648 presents an exciting new opportunity to target KAT6A in patients with ER-positive breast cancer.
Director of Medicinal Chemistry at the Monash Institute of Pharmaceutical Sciences (MIPS) and study co-author Professor Paul Stupple said, "The discovery of CTx-648, a potent, selective, and orally bioavailable KAT6A inhibitor, which has shown anti-tumor activity in preclinical models of ER-positive breast cancer, including tumors resistant to hormone therapy, is incredibly exciting.
"KAT6A is an enzyme that helps to regulate a wide variety of chemical processes in the body. However, dysregulation of KAT6A has been identified in several cancers, including breast cancer where amplification of the KAT6A gene can occur," Professor Stupple said.
Former Chief Scientific Officer of Cancer Therapeutics CRC and now the Director of MedChem Australia which sits within MIPS, Professor Brendon Monahan, commented "KAT6A amplification in breast cancer is associated with poor overall survival, with analysis of breast cancer patient datasets indicating KAT6A amplifications occur in 6%–11% of tumors.
"Tumors with KAT6A amplifications are strongly associated with shorter progression-free survival, and overall survival," said Professor Monahan.
"Hormone therapy remains the backbone for treatment of ER-positive breast cancer patients, however resistance to this line of therapy eventually develops, highlighting the need for novel therapies to target such tumors."
The team from the Center for Drug Candidate Optimization which is one of the five research themes within MIPS and led by Professor Susan Charman, played a critical role profiling the physicochemical, metabolic and pharmacokinetic properties of candidate compounds to inform medicinal chemistry design strategies for compound optimization.
Professor Charman said "Compared to previously identified KAT6 inhibitors, CTx-648 represents a marked improvement in potency, selectivity, and drug-like properties.
"CTx-648 has demonstrated robust on-target in vivo efficacy in pre-clinical models, including tumor regressions, with minimal toxicities, highlighting the promise of this novel therapy in treating breast cancer patients," said Professor Charman.
Dr. Alan Robertson, Managing Director of Canthera Discovery formally Cancer Therapeutics CRC said "This research was a team effort and the collaboration of Cancer Therapeutics CRC partners including Monash University and Pfizer was an example of how multidisciplinary teams working together can have incredible impact."
It was research led by a team from WEHI, MIPS and Cancer Therapeutics CRC that originally paved the way for the discovery of CTx-648 through their investigation into whether KAT6A and KAT6B could be a new approach to treating cancer. The study was published in Nature in 2018.
CTx-648, subsequently invented by MIPS researchers in 2018, was part of multi-million dollar licensing deal to Pfizer by the Cancer Therapeutics CRC and led to drug candidate PF-07248144, which entered Phase I clinical trials in 2020.
"There is an urgent need for new safe and effective treatments for ER-positive breast cancer and the team is excited that a KAT6A inhibitor is currently in Phase I clinical trials," said Professor Stupple.
More information: Shikhar Sharma et al, Discovery of a highly potent, selective, orally bioavailable inhibitor of KAT6A/B histone acetyltransferases with efficacy against KAT6A-high ER+ breast cancer, Cell Chemical Biology (2023). DOI: 10.1016/j.chembiol.2023.07.005