This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers discover functional compensatory effects in Treg cells
Professor Yi Sun's team at the Zhejiang University report a functional compensatory effect between the Ube2m-Rbx1 and Ube2f-Sag axes of the neddylation–Cullin-RING ligases (CRLs) system in Treg cells, using conditional KO mouse models. The team also revealed that both Ube2m-Rbx1 and Ube2f-Sag axes are essentially required for the functions of Treg cells, and elucidated mechanistically that the Rbx1/Sag-CRLs function in both neddylation-dependent and -independent manners.
Given that abnormal activation of CRLs (e.g., by enhanced cullin neddylation) are associated with many human autoimmune diseases, such as systemic lupus erythematosus, inflammatory bowel disease, and rheumatoid arthritis, this work may provide new insight and avenues for further study with ultimate goal to prevent and treatment of these diseases. The paper was published in the journal Research with the title "The functional redundancy of neddylation E2s and E3s in modulating the fitness of regulatory T cells."
Protein neddylation is a type of ubiquitination-like modification that is catalyzed by an E1-E2-E3 enzymatic cascade. Mammalian cells only have one neddylation E1, two E2s (Ube2m and Ube2f), and more than a dozen E3s. Among them, the Rbx1 E3 pairs with Ube2m to catalyze neddylation of Cullin 1-4, while the Sag/Rbx2 E3 pairs with Ube2f to catalyze neddylation of Cullin 5. Rbx1 and Sag are also two RING family members, acting as the catalytic subunit of Cullin-RING ligases (CRLs) E3 ubiquitin ligases. Neddylation on Cullins activates CRLs, the largest family of E3 ubiquitin ligases that regulate many key biological processes.
The previous studies from Dr. Sun's group found that total knockout of either Rbx1 or Sag causes embryonic death in mice at different stage of development, indicating the functional non-redundancy of these two family members. Recently, Dr. Sun's team reported that UBE2M also acts as a ubiquitin E2 to couple with Parkin-DJ-1 E3 for targeted ubiquitylation and degradation of UBE2F under the hypoxia condition, suggesting some cross-talks between these two E2s.
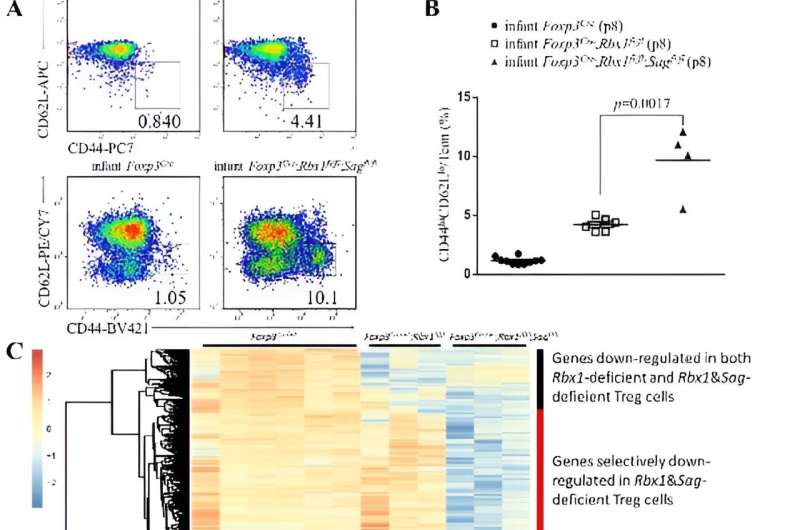
Regulatory T cells are specialized immunosuppressive CD4+-T lymphocytes, that play pivotal roles in maintaining immune homeostasis in vivo. Last year, Dr. Sun's group conditionally knocked out Ubem, Ube2F, Rbx1 or Rbx2/Sag individually in regulatory T cells (Treg cells), and found that the Ube2m-Rbx1 axis of neddylation-CRLs system is essential, whereas the Ube2f-Sag axis is dispensable for the functional maintenance of Treg cells, given that Treg cell knockout of Ube2m or Rbx1, respectively, causes significant inflammatory/autoimmune phenotypes and early death in mice; whereas the same knockout of Ube2f or Sag had no phenotype.
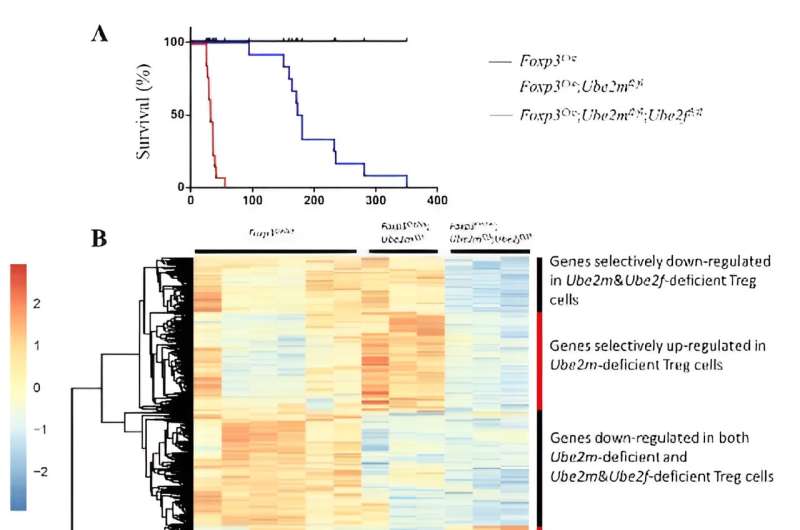
The purpose of this study is to explore whether there is functional complementarity between Ube2m-Rbx1 and Ube2f-Sag axes in Treg cells. The Sun group generated mouse strains with double knockout of both E2s Ube2M and Ube2F or E3s Rbx1 and Sag in Treg cells via Foxp3-Cre-Loxp system, respectively. The double E2 knockout mice showed robust inflammatory phenotypes with a survival period of only about 35 days, while mice that only Ube2m knocked out in Treg cells had a survival period of about 4–6 months.
Further comparison of the transcriptome changes between two E2s double knockout Treg cells and single knockout Ube2m Treg cells revealed that genes, especially inflammation-related pathways, altered in much greater degree in the former than that in the latter. The results indicate that Ube2f indeed plays a role in Treg cell function, which is, however, fully compensated by Ube2m in Treg cells.
On the other hand, the comparison of the phenotypes between double E3 knockout and single Rbx1 knockout showed similar severe inflammatory phenotypes with early-onset fatality, since the phenotype of mice with single Rbx1 knockout in Treg cells is already very severe. The difference was found only at 8-day-old young mice in which activation indicators of immune cells in double knockout mice were significantly higher than those in single knockout mice.
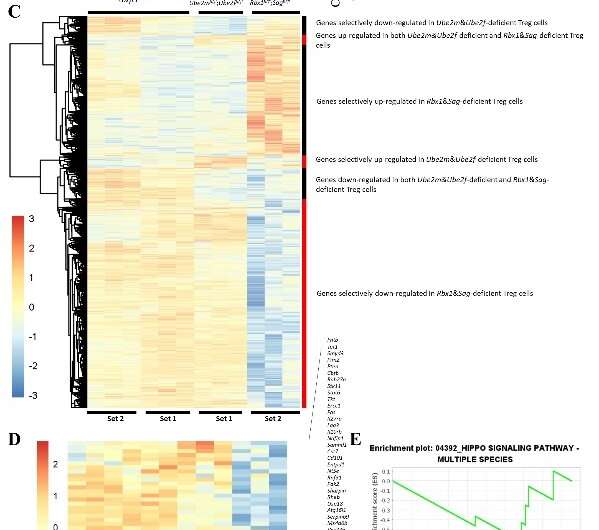
Likewise, the transcriptome changes in Treg cells with simultaneous deletion of Rbx1 and Sag were also significantly greater than those in Treg cells with single deletion of Rbx1. Thus, Sag itself also regulates Treg cell function to a certain extent; but it is fully compensated by Rbx1.
The phenotype comparison in mice with double E2 vs. double E3 knockout in Treg cells revealed severer phenotypes in double E3 knockout with much shortened life-span. The transcriptome alterations in Treg cells in the former are also much greater than those in the latter. Given that Rbx1 and Sag are dual E3 ligases that catalyze both neddylation (of cullins), and ubiquitylation (through CRL), but CRL activation requires cullin neddylation. Thus, Rbx1/Sag appears to have functions beyond neddylation-CRLs. This study further deepens the understanding of the regulatory mechanism of Rbx1/Sag-CRLs in Treg cells.
Taken together, this in vivo based study showed the compensation between the Ube2m-Rbx1 and Ube2f Sag axes of the neddylation-CRLs system in functional regulation of Treg cells. It also elucidates the role of the Rbx1/Sag-CRLs in Treg cells is partially independent of neddylation modifications, thus revealing previously unknown mechanisms of the neddylation-CRLs system in Treg cells.
It is known that abnormalities of CRL are associated with many human autoimmune diseases, and the activation of CRL requires neddylation modification. Therefore, this work provides a new angle to study and understand underlying mechanism of development of autoimmune diseases, and helps to set up the foundation for the ultimate goal in the prevention and treatment of autoimmune diseases.
More information: Di Wu et al, The Functional Redundancy of Neddylation E2s and E3s in Modulating the Fitness of Regulatory T Cells, Research (2023). DOI: 10.34133/research.0212