This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New findings about key pathological protein in Parkinson's disease open paths to novel therapies
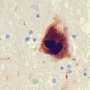
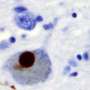
A so-called pathological protein long associated with Parkinson's disease has been found in a new study to trigger cells to increase protein synthesis, an event that eventually kills the subset of brain cells that die off in this neurodegenerative condition.
Researchers from the Johns Hopkins University School of Medicine who conducted the study say the findings offer potential new targets for treating Parkinson's disease, which affects about 1% of the U.S. population over age 60 and has no cure.
The findings were published in Science Translational Medicine. "Parkinson's disease has major impacts on quality of life for patients, but also for their caretakers and loved ones," says study leader Ted M. Dawson, M.D., Ph.D., professor in the Department of Neurology and director of the Institute for Cell Engineering at the Johns Hopkins University School of Medicine.
"We hope that research like this will provide mechanistic, molecular-based therapies that can actually slow or halt the progression of Parkinson's disease."
Parkinson's disease symptoms, including a variety of motor and cognitive deficits that worsen over time, result from the death of neurons that produce the chemical messenger dopamine. Current treatments with drugs such as L-dopa primarily focus on replacing the dopamine lost when these dopaminergic neurons die.
Over the past two decades, researchers linked these cells' death to the presence of a pathological form of alpha-synuclein, a normal protein that is abundant in brain tissue. However, how pathological alpha-synuclein causes dopaminergic neuron death has been unclear. To pin down its role, Dawson and his colleagues used proximity labeling coupled with mass spectrometry to identify proteins that might interact with pathological alpha-synuclein in both a mouse and a lab cell model of Parkinson's neurons.
They identified 100 such proteins that overlapped between these two models. When the researchers grouped the proteins by function, they found the majority play roles in ribonucleic acid (RNA) processing and translation initiation—critical processes used by cells to make new proteins.
Several of the proteins were already known to work with the mammalian target of rapamycin (mTOR), which has a dual role in both regulating protein production and breaking down proteins.
Experiments in mice genetically manipulated to over-express the pathological form of alpha-synuclein showed that it indeed caused cells to increase protein synthesis by activating mTOR.
This process was triggered, the researchers say, when the pathological alpha-synuclein bound to another protein, tuberous sclerosis complex 2 (TSC2), preventing it from connecting with yet another protein, TSC1, that keeps mTOR in check.
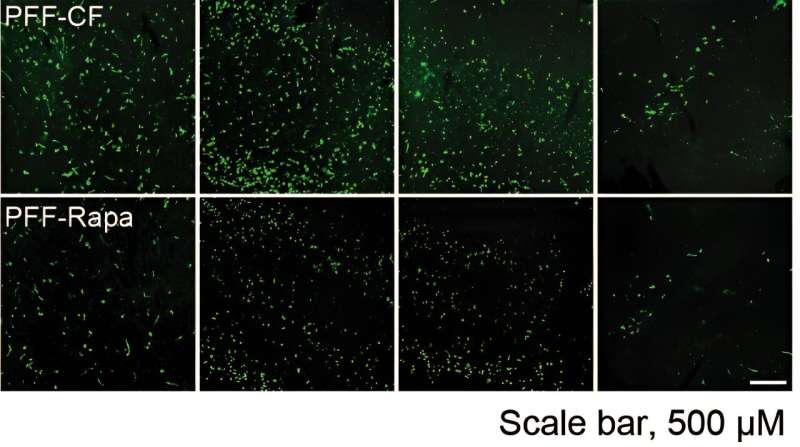
Treating the genetically engineered mice with rapamycin, a drug that targets mTOR, not only prevented excessive protein production in mice with a condition like Parkinson's disease but also eased some of the slow, halting movements and weak grip strength that are hallmarks of Parkinson's disease in people.
Dawson says it's still unclear precisely how increased protein production might harm dopaminergic neurons—the proteins might clog up key cellular pathways, or specific proteins produced in excess might be harmful to cells. He and his colleagues plan to investigate that question in future research.
In the meantime, he says, the findings point toward new targets for treating Parkinson's disease. Researchers may, for example, develop drugs that act like rapamycin—currently used as an anti-rejection and anti-cancer medication—but work specifically in the brain to save dopaminergic neurons, sparing patients unnecessary body-wide side effects. Or, it may be possible to target TSC2 to produce a similar effect.
More information: Mohammed Repon Khan et al, Enhanced mTORC1 signaling and protein synthesis in pathologic α-synuclein cellular and animal models of Parkinson's disease, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.add0499