This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Structures of Parkinson's disease-linked proteins offer a framework for understanding how they work together
Scientists at St. Jude Children's Research Hospital have revealed the complex structure of two Parkinson's disease-related proteins, both of which are implicated in late-onset cases. Leucine-rich repeat kinase 2 (LRRK2) is a protein kinase that modifies other proteins in a process called phosphorylation; Rab29, a member of the Rab GTPase family that regulates cellular trafficking, modulates the activity of LRRK2.
How Rab29 and LRRK2 work synergistically to cause Parkinson's disease remains unclear. The St. Jude researchers determined the structures of LRRK2 bound to Rab29, uncovering mysteries behind LRRK2 regulation and insights with implications for drug design.
The work was published today in Science.
Parkinson's disease is the second-most common neurodegenerative disease after Alzheimer's disease and affects 1-2% of the population older than 65 years. The genetic link to the disease is well-known, with approximately 15% of cases presenting a family history. While there is a long list of genes associated with the disease, mutation of LRRK2 is one of the most common causes. Due to its large size, structural studies on LRRK2 have been cumbersome.
"This protein is extremely challenging to work with," said corresponding author Ji Sun, Ph.D., St. Jude Department of Structural Biology.
Despite those difficulties, Sun and his team presented the first structure of full-length LRRK2 in 2021 in Cell.
"In that first paper, we got the structure of LRRK2, but that structure showed an inactive conformation," Sun explained. Proteins often have active and inactive forms, regulated by different cellular signals. Sometimes, it takes binding to another protein to trigger the structural changes that move a protein from an inactive shape to an active one. "So we started thinking, "We have one key state of LRRK2. Can we get its active conformation?'"
Cryo-electron microscopy captures the active state of LRRK2
The search for the active conformation was not as simple as adding Rab29 to LRRK2. LRRK2 can bind to other LRRK2 molecules in a process called oligomerization. This can turn a single LRRK2 monomer (one unit) into a dimer (two units)—or even larger assemblies. This meant the researchers had to search for the version that represented the active form. There was also the issue that Rab29 is located at cell membranes.
"In cells, about 90% or more LRRK2 cytosolic," explained Sun. "A very small amount is located on the membrane surface and forms large oligomers. And those are the versions that are active and functional ."
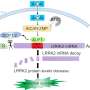
Using cryo-electron microscopy, the researchers, including first author Hanwen Zhu, Ph.D., St. Jude Department of Structural Biology, determined the first structures of the Rab29-LRRK2 complex. This included the structures of the monomer (one pair) and dimer (two pairs) but also an unexpected tetramer (four pairs).
"In this tetramer, we see the active conformation of LRRK2, but in the monomer and dimer complexes, LRRK2 is in an inactive conformation," Sun said.
Understanding the Rab29-LRRK2 complex
These findings demonstrate that LRRK2 is activated not just by the proteins with which it interacts, but also by their spatial arrangement within cells.
"We propose a transition from monomer to tetramer upon membrane recruitment," Sun explained. "Inside the cell, it's mostly inactive monomers or dimers of LRRK2. But when Rab29 recruits LRRK2 to the membrane, the local concentration of LRRK2 increases. This then facilitates the transition to tetramer, wherein LRRK2 becomes active."
What are the implications for Parkinson's disease? These structures provide researchers with an atomic-scale map to trace how the different mutations that cause Parkinson's disease affect function within this complex.
"All those mutations actually favor the active conformation, meaning they provide new interactions in the active conformation or disrupt interactions within the inactive conformation," Sun said. "The effects of the mutations can be visualized beautifully in our structures; it's very well explained."
The importance of such structural studies lies not just in the insight gained but also in their potential application for drug design. For example, the researchers also captured the structure of LRRK2 in the presence of the drug DNL201. This drug, which went through a phase 1 clinical trial, locks the protein in an active state, so it was used to validate their findings that the tetramer was indeed the active form of the complex.
"We now have an inactive conformation and an active conformation, so we can monitor the transition from the inactive to active state," Sun explained. "These structures provide much-needed insights for medicinal chemists to design novel inhibitors against LRRK2 for Parkinson's treatment."
More information: Hanwen Zhu et al, Rab29-dependent asymmetrical activation of leucine-rich repeat kinase 2, Science (2023). DOI: 10.1126/science.adi9926. www.science.org/doi/10.1126/science.adi9926