This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Scientists find a 'scarcode' common across damaged organs
Scarring goes more than skin deep. It can occur in any organ because of injury from smoking and excessive alcohol consumption or as a byproduct of chronic conditions like endometriosis, cardiovascular disease and autoimmune disorders. While scar formation is essential in wound healing, when the scarring—or fibrosis—spins out of control, it can lead to deadly consequences, contributing to almost 2 out of 10 deaths worldwide every year.
"And we still have nothing in terms of drugs to treat fibrosis," said Jacques Behmoaras, who is the deputy director of the Center for Computational Biology and an associate professor with the Cardiovascular and Metabolic Disorders Program at Duke-NUS.
Fascinated by this carefully balanced process, Behmoaras and colleagues delved deeper, zooming in on the role of macrophages, immune cells that are known to repair injured tissues and organs, to determine if the macrophages at work in the scarring of different organs share any traits that could hold the key to a new treatment approach.
"From previous studies, including our own, we had noticed a particular molecule that kept popping up in macrophages in fibrosis, a bit of a smoking gun really," systems geneticist Enrico Petretto, who leads the Center for Computational Biology and is also an associate professor with the Cardiovascular and Metabolic Disorders Program at Duke-NUS. "But rather than focus on just this signal, we wanted to approach this question in a totally unbiased manner."
That meant characterizing all the macrophages present in healthy and fibrotic human organs—an impossible task for a single lab. So instead of reaching for a pipette, the two scientists, who share a passion for computational biology, and their teams turned to big data. Using publicly available single cell sequencing datasets, they grouped macrophages by their activation signatures in as many types of organs as they could find data for.
"We created one of the largest systematic studies, if not the largest systematic study, of macrophages in fibrosis without lifting a single pipette," said Petretto, and in the process they saved costs and eliminated the need to use animal model systems.
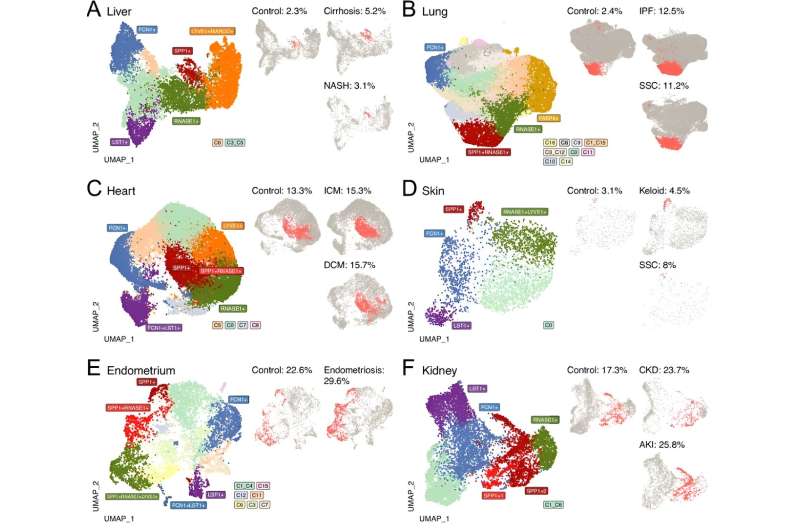
Among the methods the team applied were machine learning algorithms that helped them first to identify all cells catalogued in the raw data of the 15 studies that met their inclusion criteria. After filtering out the unwanted cell types, they ended up with 235,930 macrophages from healthy and fibrotic human heart, lung, liver, kidney, skin and endometrial tissue. To profile and cluster these by their different identities, without bias, they applied another set of algorithms.
"We found that the number of macrophages expressing SPP1 consistently increased in all fibrotic tissues," said Petretto.
Looking deeper still, they found that during the scarring process, the group of macrophages that had been labeled as SPP+ could be separated into two potentially opposing camps: one which promotes fibrosis by triggering the deposition of structural scaffolding between cells, which is essential during the early phases after an injury; and an opposing camp that will dismantle the scaffolding after the tissue has healed.
"They share the same properties but they're like yin and yang," said Behmoaras. "And it is the same in each tissue."
One organ not on the list that he would have liked to include in their paper, which was published in eLife, is muscle: "The one organ I would have liked to include is the muscle. That remains as a project to investigate in the future."
While they may have had to leave out muscle, the protocol is ready for them or anyone else to build on this analysis—a fact that the team was determined to ensure.
"To achieve this, we really had to start from scratch," recalled John Ouyang, a principal research scientist with the Cardiovascular and Metabolic Disorders at Duke-NUS, who spent some six months patiently scouring the supplementary data of the 15 papers, re-annotating them, and resolving first intra-dataset differences and then inter-dataset discrepancies.
"It took is about six months and four or five iterations before we had a protocol that it is totally reproducible. And now, seeing it working over and over—that's really exciting," he said.
Added Petretto, "We're very proud to have a protocol that anyone can run."
With this in-silico proof in hand, the team is heading back to the lab to confirm their findings, starting with the kidneys.
Petretto, who is also Associate Dean for Research Informatics at Duke-NUS, added, "We also want to investigate how to boost the 'good' population at the expense of the 'bad' guys, so that we can develop new therapies that can be effective against fibrosis in general, regardless of which organ is affected."
And it is not just the potential for new treatments of fibrotic diseases that advanced with this discovery. "If we understand scarring, we can understand healing," said Behmoaras. "If we understand healing, we can ask questions like what makes your tissue regenerate quicker?"
More information: John F Ouyang et al, Systems level identification of a matrisome-associated macrophage polarisation state in multi-organ fibrosis, eLife (2023). DOI: 10.7554/eLife.85530