This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
AI in personalized cancer medicine: New therapies require flexible and safe approval conditions
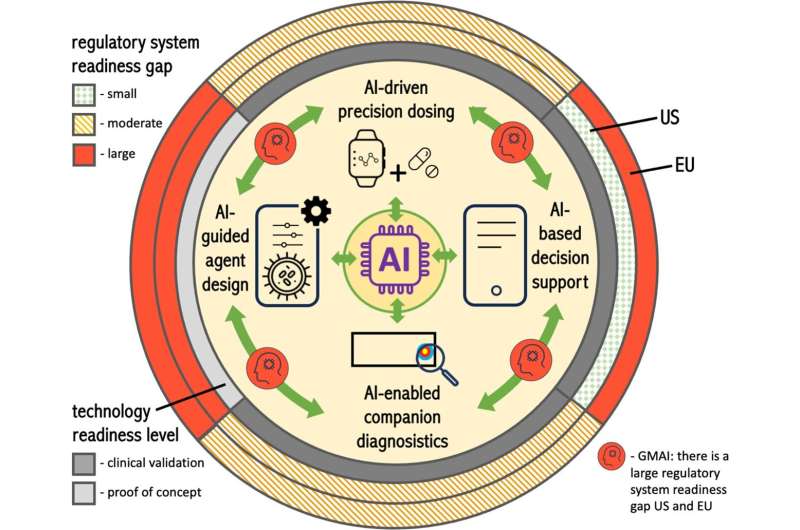
New, AI-based therapies require a flexible and safe legal framework in order to reach patients quickly and safely. In a new paper, published in npj Precision Oncology, researchers from Dresden, Leipzig, Marburg and Paris provide an overview of possible AI-based applications for personalized cancer medicine and the associated regulatory challenges. They emphasize that the current rigid and slow approval requirements impede technological progress and argue for an adaptation of the existing regulations.
The application of AI in precision oncology has so far been largely confined to the development of new drugs and has had only limited impacts on the personalization of therapies. New AI-based approaches are increasingly being applied to the planning and implementation of personalized drug and cell therapies. Therapies can be adapted to individual patients' needs—for example to improve efficacy and dosage, reduce toxicity, develop combination therapies and even personalize preclinical cell therapies regarding their molecular properties.
AI-based health care is developing continuously and with increasing speed. It can support doctors with decision-making and therapy planning as well as in early multi-cancer precision diagnostics. Other potential applications include the design of new types of personalized medical products, drug companion apps for patients and the use of so-called "digital twins." The latter use patient data in almost real-time to enable more precise diagnosis by means of simulation and modeling and to adapt treatments to individual requirements.
Advancing these products through regulatory pathways is enormously challenging. They combine technologies governed by different legal frameworks and regulatory bodies and are so novel that they are not well dealt with in current legislation. It can already be anticipated that the current approval conditions will make rapid clinical application difficult.
Making approval processes more agile in the future
The publication identifies two large challenges: Legislators and regulatory bodies underestimate the importance of the developing technologies in this area as well as the extent of required regulatory change to make approval processes more agile in the future. "The current regulations are a de facto blocker to AI-based personalized medicine. A fundamental change is needed to solve this problem," says Stephen Gilbert, Professor of Medical Device Regulatory Science at the Else Kröner Fresenius Center for Digital Health at TU Dresden and University Hospital Carl Gustav Carus Dresden.
The researchers therefore suggest, among other things, updating risk-benefit assessments for highly personalized treatment approaches. Solutions already established in the U.S. could also be adopted in the EU for certain classes of low-risk decision support for doctors. The authors further suggest approaches to allow digital tools on market to be safety adaptable in a more flexible manner and to establish suitable test platforms for on-market monitoring. Multi-layered approaches would help to spread the load of oversight and make evaluation more relevant to patient safety.
More information: Bouchra Derraz et al, New regulatory thinking is needed for AI-based personalised drug and cell therapies in precision oncology, npj Precision Oncology (2024). DOI: 10.1038/s41698-024-00517-w