This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study reveals critical role of FAM3C in breast cancer progression
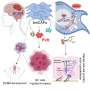
A study conducted by Professor Jiyoung Park and her research team in the Department of Biological Sciences at UNIST has identified FAM3C, a metabolism-regulating signaling molecule produced by cancer-associated adipocytes (CAAs), as a key regulator of breast cancer progression within the tumor microenvironment (TME).
The findings, published in Cancer Research, shed light on the potential for targeted therapies in the treatment of breast cancer.
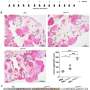
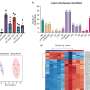
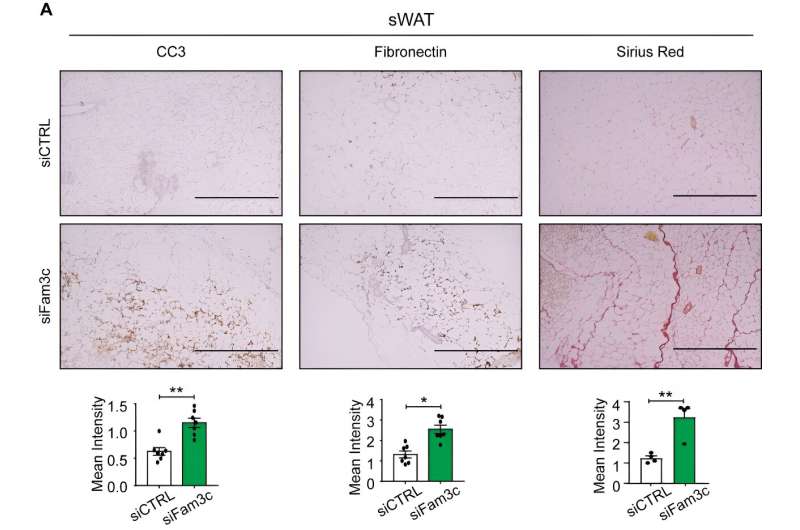
The study demonstrates that overexpression of FAM3C in cultured adipocytes significantly reduces cell death in both adipocytes and co-cultured breast cancer cells, while suppressing markers of fibrosis. Conversely, depletion of FAM3C in CAAs leads to adipocyte-mesenchymal transition (AMT) and increased fibrosis within the TME. The research team also discovered that breast cancer cells stimulate the expression of FAM3C in adipocytes through TGF-β signaling, which can be inhibited by a TGF-β-neutralizing antibody.
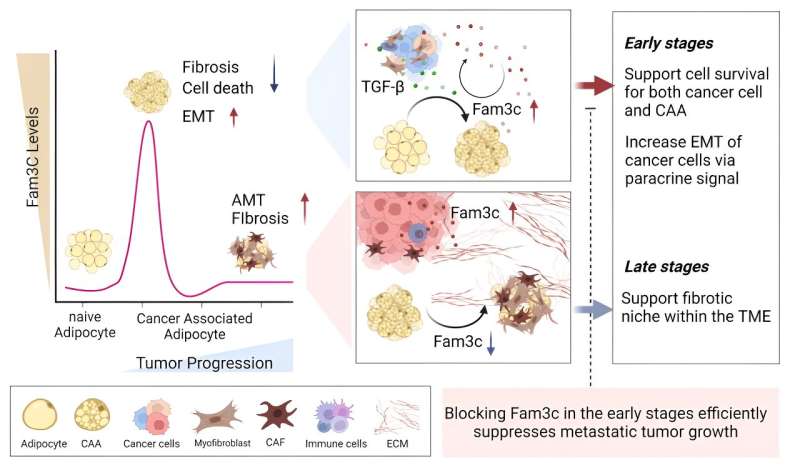
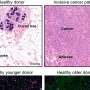
In a genetically engineered mouse model of breast cancer, early knockdown of FAM3C in CAAs significantly inhibited primary and metastatic tumor growth. Furthermore, elevated levels of circulating FAM3C were observed in patients with metastatic breast cancer compared to those with non-metastatic breast cancer.
"These findings suggest that therapeutic inhibition of FAM3C expression in CAAs during early tumor development could hold promise as a novel approach in the treatment of patients with breast cancer," said Professor Jiyoung Park. "Understanding the role of cancer-associated adipocytes and their secretory molecules, such as FAM3C, opens up new avenues for the development of early diagnosis markers and targeted treatments for breast cancer."
More information: Sahee Kim et al, FAM3C in cancer-associated adipocytes promotes breast cancer cell survival and metastasis, Cancer Research (2023). DOI: 10.1158/0008-5472.CAN-23-1641