This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study offers new clues into the causes of post-infectious chronic fatigue syndrome
In a detailed clinical study, researchers at the National Institutes of Health have found differences in the brains and immune systems of people with post-infectious myalgic encephalomyelitis/chronic fatigue syndrome (PI-ME/CFS). They also found distinct differences between men and women with the disease. The findings were published in Nature Communications.
"People with ME/CFS have very real and disabling symptoms, but uncovering their biological basis has been extremely difficult," said Walter Koroshetz, M.D., director of NIH's National Institute of Neurological Disorders and Stroke (NINDS).
"This in-depth study of a small group of people found a number of factors that likely contribute to their ME/CFS. Now researchers can test whether these findings apply to a larger patient group and move towards identifying treatments that target core drivers of the disease."
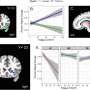
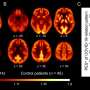
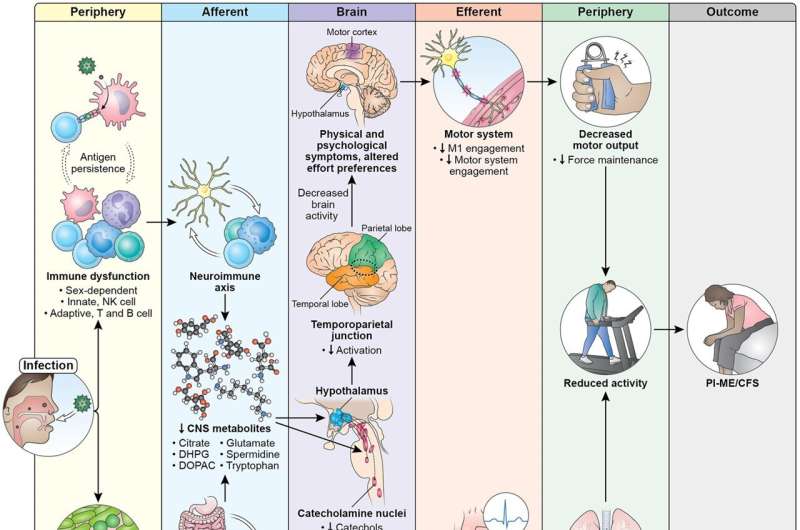
A team of multidisciplinary researchers discovered how feelings of fatigue are processed in the brains of people with ME/CFS. Results from functional magnetic resonance imaging (fMRI) brain scans showed that people with ME/CFS had lower activity in a brain region called the temporal-parietal junction (TPJ), which may cause fatigue by disrupting the way the brain decides how to exert effort.
They also analyzed spinal fluid collected from participants and found abnormally low levels of catecholamines and other molecules that help regulate the nervous system in people with ME/CFS compared to healthy controls. Reduced levels of certain catecholamines were associated with worse motor performance, effort-related behaviors, and cognitive symptoms. These findings, for the first time, suggest a link between specific abnormalities or imbalances in the brain and ME/CFS.
"We think that the immune activation is affecting the brain in various ways, causing biochemical changes and downstream effects like motor, autonomic, and cardiorespiratory dysfunction," said Avindra Nath, M.D., clinical director at NINDS and senior author of the study.
Immune testing revealed that the ME/CFS group had higher levels of naive B cells and lower levels of switched memory B cells—cells that help the immune system fight off pathogens—in blood compared to healthy controls. Naive B cells are always present in the body and activate when they encounter any given antigen, a foreign substance that triggers the immune system.
Memory B cells respond to a specific antigen and help maintain adaptive or acquired immunity. More studies are needed to determine how these immune markers relate to brain dysfunction and fatigue in ME/CFS.
To study fatigue, Dr. Nath and his team asked participants to make risk-based decisions about exerting physical effort. This allowed them to assess the cognitive aspects of fatigue, or how an individual decides how much effort to exert when given a choice.
People with ME/CFS had difficulties with the effort choice task and with sustaining effort. The motor cortex, a brain region in charge of telling the body to move, also remained abnormally active during fatiguing tasks. There were no signs of muscle fatigue. This suggests that fatigue in ME/CFS could be caused by a dysfunction of brain regions that drive the motor cortex, such as the TPJ.
"We may have identified a physiological focal point for fatigue in this population," said Brian Walitt, M.D., M.P.H., associate research physician at NINDS and first author of the study. "Rather than physical exhaustion or a lack of motivation, fatigue may arise from a mismatch between what someone thinks they can achieve and what their bodies perform."
Deeper analyses revealed differences between men and women in gene expression patterns, immune cell populations, and metabolic markers. Males had altered T cell activation, as well as markers of innate immunity, while females had abnormal B cell and white blood cell growth patterns. Men and women also had distinct markers of inflammation.
"Men and women were quite divergent in their data, and that tells you that ME/CFS is not one-size-fits-all," said Dr. Nath. "Considering male and female immune differences in ME/CFS, the results may open up new avenues of research that could provide insight into other infection-associated chronic diseases."
The study, which was conducted at the NIH Clinical Center, took a comprehensive look at ME/CFS that developed after a viral or bacterial infection. The team used state-of-the-art techniques to examine 17 people with PI-ME/CFS who had been sick for less than five years and 21 healthy controls.
Participants were screened and medically evaluated for ME/CFS over several days and underwent extensive tests, including clinical exams, fMRI brain imaging, physical and cognitive performance tests, autonomic function tests, skin and muscle biopsies, and advanced analyses of blood and spinal fluid.
Participants also spent time in metabolic chambers where, under controlled conditions, their diet, energy consumption, metabolism, sleep patterns, and gut microbiome were evaluated. During a second visit, they completed a cardiopulmonary exercise test to measure the body's response to exercise.
Many studies have identified immune, microbiome, and other abnormalities in ME/CFS, but the results tend to be inconsistent and exactly how these markers relate to or cause fatigue and other symptoms is unknown.
By using a rigorous phenotyping approach to pull out meaningful differences, this study helps validate prior results and may identify new ways to target the brain or immune system therapeutically.
The highly collaborative project involved 75 investigators across 15 institutes and centers in the NIH Intramural Research Program, and at national and international institutions. Dr. Nath and his colleagues plan to publish additional findings from the data that was collected during this study.
More information: Walitt, B. et al, Deep phenotyping of Post-infectious Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Nature Communications (2024). DOI: 10.1038/s41467-024-45107-3. www.nature.com/articles/s41467-024-45107-3