This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
COVID-19 antiviral treatment should be taken for longer, says study
The currently recommended five-day course of molnupiravir, an antiviral treatment, may not be long enough to treat COVID-19, according to a new paper involving UCL researchers.
The study, published in Nature Communications, was conducted as part of PANORAMIC, an ongoing clinical trial evaluating potential treatments for COVID-19. In this virology sub-study, the researchers analyzed viral samples provided by 577 trial participants from across the U.K. who were randomly assigned to receive either a five-day course of molnupiravir or usual care without antiviral treatments.
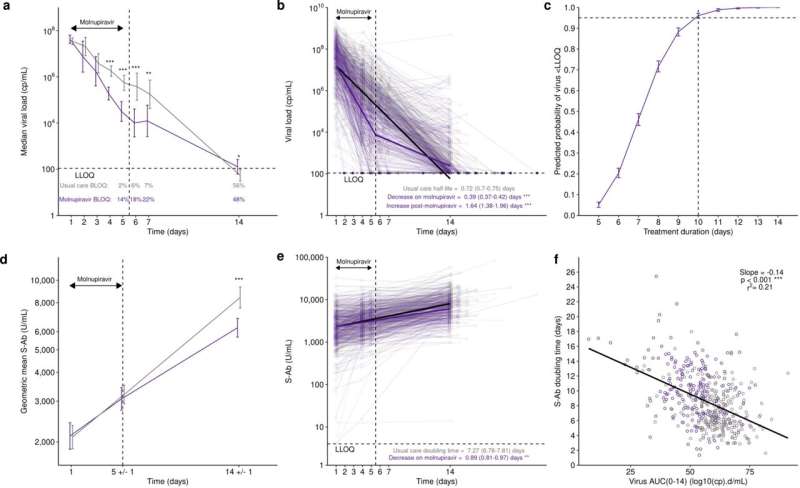
The researchers found that molunpiravir initially lowered patients' viral load more quickly than usual care, with 14% of molnupiravir patients having cleared the virus by day five, compared to only 2% of the usual care group.
However, post-treatment the rate of viral decline in molnupiravir-treated participants significantly slowed. Nine days after treatments, only 48% of molnupiravir-treated patients had cleared the virus, compared to 56% of the patients receiving usual care.
Furthermore, virus from these later samples contained more mutations than those seen in the usual care group. The researchers were able to grow some of these mutated viruses in the lab from samples taken up to nine days post-treatment, which suggests they could potentially be passed from patients who have taken molnupiravir to others.
Lead author Professor Joseph Standing (UCL Great Ormond Street Institute of Child Health) said, "We know that molnupiravir works by introducing errors into the SARS-COV-2 virus—which causes COVID-19. Mutated variations of the virus often cannot replicate well and this is why we saw faster viral load decline.
"However, the fact the virus remains in patients after the five-day molnupiravir treatment course indicates the course is not long enough to clear the virus from the body.
"It is possible that these patients could be spreading virus that has mutated and, if large numbers of patients are treated in this way, it is possible new variants, that help the virus evade the human immune system more effectively, may arise."
The five-day course, say the authors, is not long enough to completely clear the virus. They suggest a longer course of treatment is trialed to see if this eliminates the virus from the body and limits the potential for patients to continue to spread the infection after the end of the treatment.
The authors also looked at the body's immune response to the virus—specifically, the "spike protein" antibody levels after infection and treatment. They found that the group who had taken molnupiravir had 6,200 U/mL of antibodies after the treatment ended, compared to 8,400 U/mL in the group that received usual care.
This could be because the treatment lowered the initial viral load, so the immune system didn't need to have such a strong response and produced fewer antibodies as a result. It potentially means molnupiravir-treated participants would be susceptible to re-infection sooner than the patients who received usual care.
"These findings raise important questions about the impact of molnupiravir on patients' immunity," said Dr. Oliver van Hecke, Senior Clinical Research Fellow at the University of Oxford and co-author of the study. "Even if taking molnupiravir for longer fully gets rid of the virus, some patients might still develop weaker protection against catching it again in the future.
"It would be useful to explore whether molnupiravir together with other antiviral drugs in combination better eliminates the virus, and ensure patients build up good immunity against it. This could help prevent treated patients from catching it again soon after and also reduce the chance of passing on mutated viral strains."
These findings, say the authors, could help policymakers shape future antiviral treatment strategies and should inform future trials for COVID-19 treatments.
More information: Joseph F. Standing et al, Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications (2024). DOI: 10.1038/s41467-024-45641-0