This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
The hepatitis E virus: New insights into targeted treatment and diagnosis
Common symptoms of liver inflammation caused by hepatitis E viruses (HEV) include fever, abdominal pain, pale stools, nausea, and jaundice. Individuals at risk for this infection include persons with weakened immune systems (immunocompromised) as well as pregnant women. Immunocompromised patients often suffer from chronic infections, which is a larger problem for the Global North.
Pregnant women often suffer from severe cases (fulminant hepatitis). This type of hepatitis is associated with mortality rates of up to 30 percent and occurs primarily in the Global South. The geographical differences can be explained by the fact that zoonotic, food-borne HEV strains (genotypes 3 and 4) circulate predominantly in the north, while the mainly water-borne genotypes 1 and 2 are present in the south.
So far no vaccines against HEV have been authorized in Europe. Although there are medicines that are used to treat infections, the treatment options are still limited and associated with strong side effects or the development of resistance. This is also due to a lack of understanding of large parts of the viral life cycle.
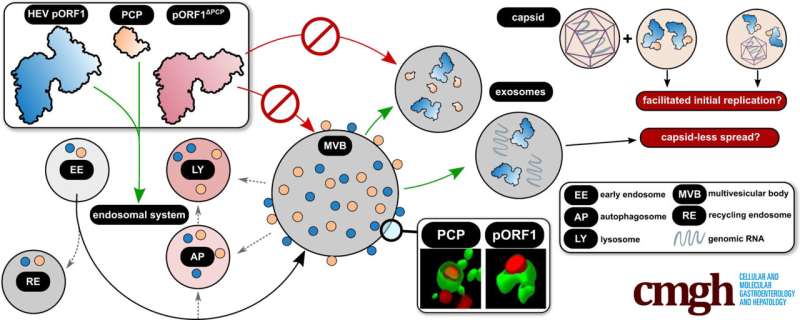
Viral polyprotein pORF1—central to the production of the viral genome and yet almost unknown
The reproduction of HEV's genetic information (genome replication) is mediated via the viral polyprotein pORF1. This protein consists of multiple domains. A protein domain is a stably folded structure within a protein that is functionally and structurally independent of adjacent protein segments.
pORF1 is the central protein for replication (replicase), which means it is responsible for the reproduction of the virus' genetic information. However, little else is known about pORF1, including its exact location within infected cells.
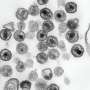
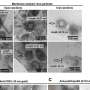
Researchers led by Dr. Mirco Glitscher in the working group of Professor Eberhard Hildt, head of the Virology Division at the Paul-Ehrlich-Institut, focused on this important unknown HEV element. In their research, they used confocal laser scanning microscopy to analyze the subcellular localization of pORF1 and its individual domains, which were generated and cloned on the basis of a structural prediction of the viral replicase.
The exosomes released from the cells were isolated using ultracentrifugation and analyzed by isopycnic (separation according to the same density) density gradient centrifugation. The separated particles were then examined more closely by fluorimetry, Western blot analyzes, or RT-qPCR.
The vesicular system as the cornerstone of virus reproduction
The research team found that pORF1 accumulates in the vesicle system of the cell—the endosomal system—and primarily in multivesicular bodies (MVBs). These structures are central to exosome formation and have hitherto been regarded as only a host structure for the release of viral HEV particles. The presence of the viral replicase here was identified as being dependent on a pORF1 domain, the PCP (papain-like cysteine protease).
The research team thus found that the viral replicase is also released via exosomes. This process is mediated by the viral protease that is part of the replicase. As a result, viral genomes are released via this route even in the absence of virus particles.
The findings indicate that pORF1 enters MVBs in a PCP-dependent manner, which is followed by exosomal release. Thus the release of the virus and replication, the reproduction of genetic information, may be spatially coupled. This could facilitate the spread of viral infection, as genomes that enter the cell during re-infection can quickly encounter exosome-transmitted pORF1 and be replicated. In addition, the data collected indicates that the capsid is not necessarily required for the release of genetic material.
The exosomes and associated protein structures could be suitable targets for therapeutics against HEV, because this could possibly prevent both viral replication and viral release. The capsid-independent release of the HEV genomes certainly has an impact on diagnostics, since it has been hitherto assumed that there is a correlation in the amount of virus particles and viral genomes.
However, the relevance of the presence of genomic RNA in exosomes without a capsid when it comes to diagnostic procedures must still be investigated.
The research is published in the journal Cellular and Molecular Gastroenterology and Hepatology.
More information: Mirco Glitscher et al, The Protease Domain in HEV pORF1 Mediates the Replicase's Localization to Multivesicular Bodies and Its Exosomal Release, Cellular and Molecular Gastroenterology and Hepatology (2024). DOI: 10.1016/j.jcmgh.2024.01.001