This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Immune networks in tumors found to prime responses to personalized immunotherapy
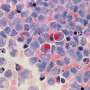
Through an analysis of tumor samples collected over time from patients with advanced melanoma, a Ludwig Cancer Research study has identified a set of preexisting conditions in tumors that predict whether such patients are likely to respond to a personalized immunotherapy known as adoptive T-cell therapy (ACT) using tumor-infiltrating lymphocytes (TIL).
Led by Ludwig Lausanne's David Barras, Eleonora Ghisoni, Johanna Chiffelle, Denarda Dangaj Laniti, and Branch Director George Coukos and reported in Science Immunology, the study also describes biomarkers that, with further vetting, could help clinicians select patients for TIL-ACT. In this therapy, TIL—which kills cancerous cells—is isolated from a patient, expanded in culture, and then reinfused into the patient as a treatment.
"Given the aggressiveness of advanced melanoma, the potential value of TIL-ACT for patients who respond to it after failing immune checkpoint blockade immunotherapy and other available lines of therapy can't be overstated," said Coukos.
"The question, of course, is who those people are, and since only a fraction of patients currently benefit from the experimental therapy, it is vitally important to be able to quickly identify those who are unlikely to respond so that they can be quickly offered alternative treatments. Our study has taken a big step toward making that possible."
The Lausanne Branch of the Ludwig Institute for Cancer Research is developing several strategies for personalized immunotherapies, ranging from cancer vaccines to personalized adoptive cell therapies (ACT) for various cancers, including TIL-ACT.
To explore how the tumors differed between patients who responded to treatment and others, the researchers collected tumor samples from patients before therapy started and then at various time points after they had undergone TIL-ACT treatment.
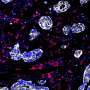
They then examined differences between the global gene expression patterns of individual cancerous and noncancerous cells and conducted additional molecular analyses of cellular features and, most notably, interactions between cells in the context of their location within the tumors.
"Through these analyses," Barras explained, "we discovered the underlying tumor cell biology and characteristics of the tumor microenvironment that mediate responses to ACT."
The researchers show that tumors that responded best to TIL-ACT were those that were most riddled with mutations—and therefore coruscated with neoantigens likely to be recognized by CD8+ (or killer) T cells. Further, as might be expected, the killer T cells in these tumors were in states with a potential for intense anti-tumor activation.
"Our most significant finding in this context was that tumors with preexisting networks of immune cells were the ones most primed to respond to TIL-ACT, and patients whose tumors featured such networks were the ones who responded best to therapy," said Dangaj. "That included a pair of patients enrolled in the trial whose tumors were completely cleared by the treatment."
Those networks consisted of killer T cells in close association with myeloid cells—dendritic cells and macrophages—that "present" antigens to killer T cells to guide them to their targets. These cells also hyperactivate them by binding a protein known as CD28 on the TILs to boost and sustain their functionality and secrete other T cell-stimulating factors. Moreover, these myeloid cells, like the killer T cells, were themselves in an activated state in responsive patients.
The researchers found in examining tumor samples collected after treatment that successful TIL-ACT therapy further expanded and activated these immune cell networks. Macrophages additionally expressed a molecule named CXCL9 that likely bolsters stimulatory interactions with T cells.
Notably, the findings reflect discoveries Coukos, Dangaj, and colleagues have made in studying the responsiveness of ovarian tumors to an approved immunotherapy known as PD-1 checkpoint blockade.
"Aside from the value of improved patient stratification, our discoveries on the cell and molecular biology of tumors that respond to TIL-ACT could help us devise treatment strategies to 'precondition' patients to respond to this therapy," said Coukos. "That is a very exciting possibility and one we are eager to pursue."
More information: David Barras et al, Response to tumor-infiltrating lymphocyte adoptive therapy is associated with preexisting CD8 + T-myeloid cell networks in melanoma, Science Immunology (2024). DOI: 10.1126/sciimmunol.adg7995