February 5, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A neural mechanism that could underlie fasting-mediated immune regulation
Fasting, the voluntary abstention from eating and sometimes drinking for a set time, has become increasingly widespread, as some studies have found that it could boost the immune system and help prevent the development of some diseases. There are now several smartphone applications on which people can record their periods of intermittent fasting and find out more about the potential benefits of fasting.
Researchers at University of Science and Technology of China recently carried out a study on mice aimed at better understanding the neural processes underlying the reported effects of fasting on the immune system. Their findings, published in Nature Neuroscience, suggest that fasting could specifically promote the neuronal control of inflammation and influence the distribution of T-cells, a class of white blood cells that contribute to fighting germs and protecting the body from diseases.
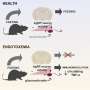
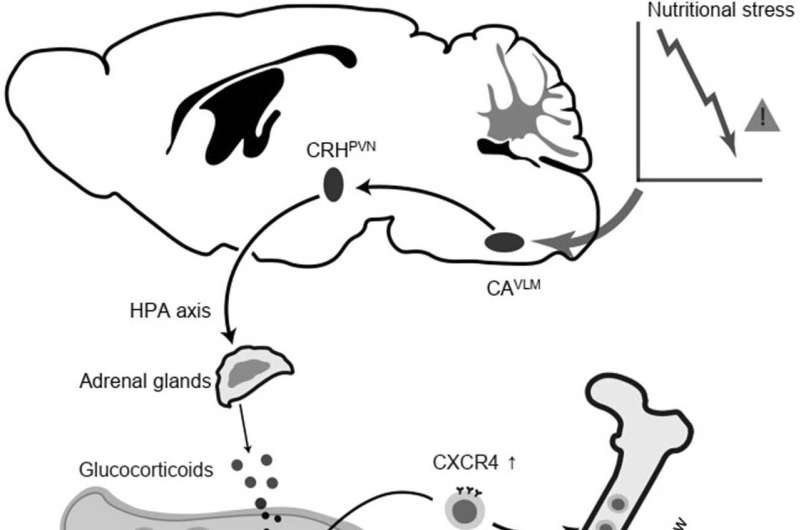
"Dietary fasting markedly influences the distribution and function of immune cells and exerts potent immunosuppressive effects," Liang Wang, Mingxiu Cheng and their colleagues wrote in their paper. "However, the mechanisms through which fasting regulates immunity remain obscure. We report that catecholaminergic (CA) neurons in the ventrolateral medulla (VLM) are activated during fasting in mice, and we demonstrate that the activity of these CA neurons impacts the distribution of T cells and the development of autoimmune disease in an experimental autoimmune encephalomyelitis (EAE) model."
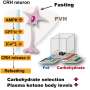
As part of their study, Wang, Cheng and their collaborators set out to test a key hypothesis, namely that catecholaminergic (CA) neurons in a brain region known as the ventrolateral medulla (VLM), also referred to as CAVLM neurons, play a part in fasting-mediated changes to the immune system of mammals. These neurons have previously been found to mediate the responses elicited in the body as a result of nutritional stress, while also influencing inflammation.
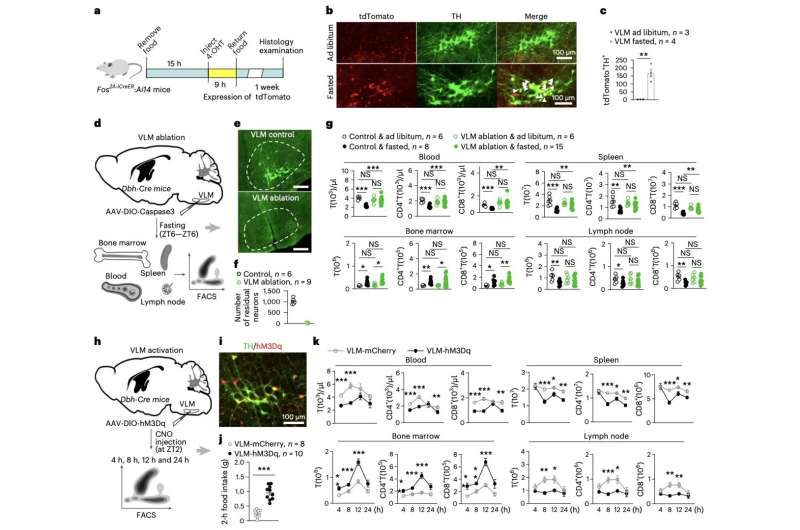
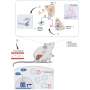
To test this hypothesis, they first examined the activity of these neurons in mice that were fed regularly and in mice that were not fed for 24 hours. In addition, they then selectively ablated and activated these neurons, to observe how their intervention influenced other neural mechanisms, as well as the course of autoimmune diseases, in mice.
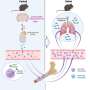
"Ablation of CAVLM neurons largely reversed fasting-mediated T cell redistribution," Wang, Cheng and their colleagues wrote. "Activation of these neurons drove T cell homing to bone marrow in a CXCR4/CXCL12 axis-dependent manner, which may be mediated by a neural circuit that stimulates corticosterone secretion. Similar to fasting, the continuous activation of VLM CA neurons suppressed T cell activation, proliferation, differentiation and cytokine production in autoimmune mouse models and substantially alleviated disease symptoms."
The findings gathered by this team provide some new valuable insights into the neural mechanisms that could drive some of the previously observed benefits of intermittent fasting. Specifically, the team identified a neural mechanism that could underlie the fasting-mediated regulation of immune cells.
The neural mechanism they uncovered, linked to the activity of CAVLM cells, appears to control the distribution of T-cells, which are already known to be linked to inflammation.
This recent work could pave the way for additional studies focusing on the newly identified neural mechanism, which could collectively contribute to the development of fasting-related therapeutic interventions for preventing and treating autoimmune diseases.
More information: Liang Wang et al, Fasting-activated ventrolateral medulla neurons regulate T cell homing and suppress autoimmune disease in mice, Nature Neuroscience (2024). DOI: 10.1038/s41593-023-01543-w
© 2024 Science X Network