This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Outlining the future of theranostics in neurooncology
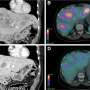
Nuclear medicine has the potential to change the landscape of theranostics in neurooncology, according to a new article published in The Journal of Nuclear Medicine (JNM). With recent advances in techniques to permeate the brain-blood barrier (BBB), the prospect of using radiopharmaceuticals to treat brain tumors such as meningiomas, gliomas, brain metastases, and pediatric brain tumors is promising.
"In the last decade, we have observed a huge step forward in treatment options for a wide range of tumors in terms of both survival and quality of life. However, therapeutic approaches to brain tumors remain a challenge, with considerable limitations regarding delivery of drugs," stated the article's authors. "There has been renewed and increasing interest in translating the popular theranostic approach well known from prostate and neuroendocrine cancer to neurooncology. Although far from perfect, some of these approaches show encouraging preliminary results."
In this article, the authors have provided a general overview of the use of theranostics for four areas of neurooncology and provided perspectives on future research needs. The article focuses on meningiomas, gliomas, brain metastases, and pediatric brain tumors.
Meningiomas are the brain tumors for which peptide receptor radionuclide therapy (PRRT) has been most performed. It is currently used in meningiomas that cannot be treated with surgery or conventional radiation therapy regardless of their grade. Most of the available data on PRRT in meningiomas are from patients at a late stage of the disease when the efficacy of the treatment is potentially limited.
It might be advantageous, state the authors, to start PRRT earlier in the disease course before patients develop treatment-refractory, progressive, and extensive disease. Future studies should include the development of criteria for appropriate use of PRRT in specific subtypes and the determination of efficacy in randomized prospective trials, as well as focus on treatment combinations.
Gliomas are the most common malignant brain tumors, with around 80% of tumors considered high-grade. Many potential theranostic targets for gliomas have been investigated, with variable but mostly discouraging results. Future studies should focus on patient selection and using multimodal approaches combining theranostic agents with techniques enhancing BBB or blood-tumor barrier (BTB) permeability.
Current therapeutic options for brain metastases consist of a combination of surgery, external radiation therapy, and targeted and immune-modulating therapies. As primary cancer control is advancing dramatically, brain metastases across many cancer types occur more frequently, and more effective therapies are needed.
Radionuclide therapy for brain metastases has been scarcely investigated; however, an advantage of radionuclide therapy over immune therapy is that the effective targeting of all lesions can be visualized using intratherapy scanning. Moreover, the effective targeting of brain metastases can be monitored by PET imaging. These features might translate into an advantage over the current standard of care in terms of clinical benefit.
Pediatric brain tumors are the most frequent solid malignancy in childhood and account for 20% of all pediatric tumors. Surgery is the mainstay in many pediatric brain tumors and can be combined with external radiation therapy or chemotherapy, although this is not ideal for young patients. A relatively large quantity of literature is available on theranostic approaches to the use of radioligands in pediatric neurooncology; the best-documented and most promising approach is the use of intracranioventricular 131I-omburtamab for treatment of leptomeningeal disease.
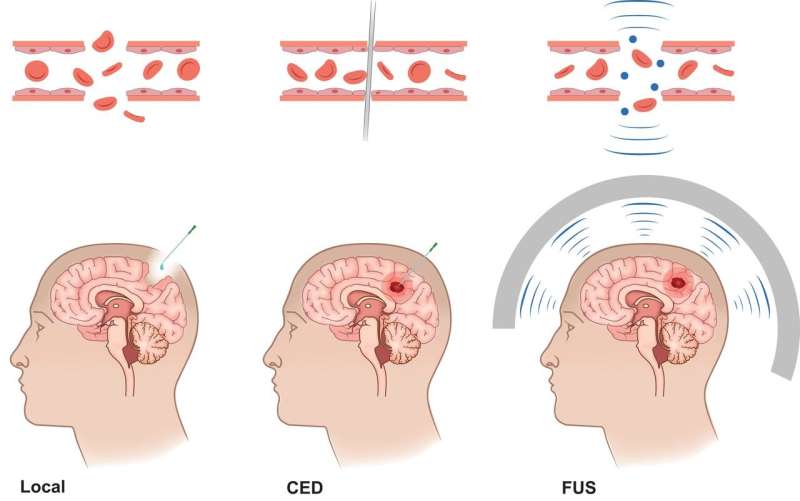
The main obstacle in neurooncology compared with other solid tumors is getting therapeutics through the BBB and the BTB. Several strategies have been developed to bypass them, and these potential pathways can allow therapeutics to be directly administered to the tumor or into surgical or anatomical cavities.
"The success of most theranostic agents will depend on the development and clinical implementation of principles that increase the permeability of the BBB," state the authors. "Here, nuclear medicine techniques can aid and potentially speed development by enabling visualization and verification of the principle."
More information: Nelleke Tolboom et al, Theranostics in Neurooncology: Heading Toward New Horizons, Journal of Nuclear Medicine (2023). DOI: 10.2967/jnumed.123.266205