This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers find response to ketamine depends on opioid pathways, but varies by sex
Ketamine, increasingly popular as a treatment for depression and pain, is often prescribed as an alternative to addictive opioids. But the data has been mixed on whether ketamine's effects rely on similar brain pathways as opioids. New research by Stanford Medicine scientists suggests that the confusion may have to do with an overlooked factor—sex.
In a study of rats, the researchers found that blocking opioid receptors in the brain extinguished the effects of ketamine—but only in male rats. Female rats' reaction to ketamine remained largely the same.
"The main thing we're interested in is whether there is a functional interaction between ketamine and the opioid system," said Raag Airan, MD, Ph.D., assistant professor of radiology, referring to receptors in the brain that bind to opioids. Airan is the senior author of a study published Jan. 30 in Nature Communications describing the findings. The study confirms that ketamine and opioids do share brain pathways, at least in some circumstances.
It's more evidence that "ketamine's a very weird drug," Airan said, and that we need to be cautious in the excitement to prescribe ketamine for a wide variety of conditions.
Ketamine is generally known as an NMDA receptor antagonist, meaning it blocks receptors for the neurotransmitter glutamate—though other NMDA receptor antagonists do not have ketamine's therapeutic effects. In 2018, a small clinical trial by Stanford Medicine researchers found that ketamine's antidepressant effects could be suppressed by first giving patients naltrexone, a drug that blocks opioid receptors. The study kicked off controversy in the field.
Airan, whose lab has been developing ways to precisely deliver ketamine in the brain, was inspired to replicate the clinical trial in rats, which would allow a closer look at what was happening in the brain.
Results may vary
Airan's team initially was not looking for sex differences. Tommaso De Ianni, Ph.D., a former postdoc in Airan's lab and lead author of the study, tested one group of rats and found that, indeed, naltrexone appeared to block ketamine's effects on brain areas related to depression and reward processing. But to his dismay, when he repeated the experiment in a second group, naltrexone appeared to have no effect. Combing through the details of the experiments, De Ianni and Airan realized there was only one difference: The first group were all male rats and the second group all female.
"It was honestly a surprise," Airan said. "We saw a very clear sex difference. The change was pretty prominent in the males, and just not there in the females."
To investigate this unexpected discrepancy, the researchers designed a trial to compare male and female rats receiving three different drug combinations: naltrexone followed 10 minutes later by ketamine, saline (as a placebo) followed by ketamine, and naltrexone followed by saline. It was a crossover trial, meaning each animal would receive all three combinations in random order, separated by a one-week waiting period.
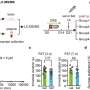
Using functional ultrasound imaging, a relatively new technique that maps, in high resolution, brain activity in awake animals, the researchers saw that in male rats, naltrexone blocked ketamine-induced changes in neural activity, including in brain areas involved in depression, such as the medial prefrontal cortex, and reward processing, such as the nucleus accumbens and lateral habenula. In female rats, naltrexone pretreatment caused no statistically significant variations in ketamine-induced brain activity.
The researchers also discovered that if they surgically removed the testes of male rats—thereby eliminating their source of testosterone—naltrexone pretreatment lost its effect, and male rats responded similarly to female rats.
The team then compared physiological changes in the brain. Ketamine is known to reverse the loss of synapses, or neuronal connections, often seen in depression and stress-related disorders—a phenomenon the researchers confirmed in both male and female rats. When male rats were pretreated with naltrexone, however, ketamine lost its synapse-restoring powers. Again, the female rats showed no significant changes.
The researchers also observed differences in behavior. Repeated ketamine intake typically causes more physical activity in animals, known as locomotor sensitization. This behavior, too, was blocked in male rats treated with naltrexone prior to ketamine, but not in female rats.
All these results point to ketamine relying, at least in part, on the opioid system, Airan said. "We think there is convincing imaging, physiological and behavioral data now supporting that, indeed, ketamine and the opioid system are interacting, and that there's functional relevance."
These interactions would be important to consider in treating patients. "What if somebody comes in with depression, but they also are taking opioids for chronic back pain?" Airan asked. "Would they respond differently to ketamine treatment for depression?
Females compensate
As to how female rats still experience ketamine's effects with the opioid blocker, the researchers think their brains are able to compensate by simply growing more opioid receptors. When they compared the brains of male and female rats treated repeatedly with naltrexone, they found that females had increased the density of opioid receptors, side-stepping the blockade. Perhaps, in male rats, testosterone somehow limits their brains' ability to grow more opioid receptors.
"It's possible that the testosterone is blocking this compensatory response," Airan said, "meaning the male brain without testosterone would compensate, but with testosterone on board, it does not."
His team is planning future studies to explore this very question, among many others raised by the new study. What would happen if they gave male rats estrogen, for example, or female rats testosterone?
Not accounting for sex may have contributed to the inconsistent data on ketamine's interaction with the opioid system, Airan said. Few other studies on ketamine have focused on sex as a variable. In preclinical experiments, though there are efforts to include more female animals, male animals are still used more frequently. Most ketamine studies on people have too few participants to tease out a statistically significant sex difference.
"In our clinical trial designs, we have to be powered to consider biological variables like sex, and we have to be looking," Airan said. "We can't just assume, 'Oh, it's probably fine.'"
More information: Tommaso Di Ianni et al, Sex dependence of opioid-mediated responses to subanesthetic ketamine in rats, Nature Communications (2024). DOI: 10.1038/s41467-024-45157-7