This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Cell division, DNA repair and cancer progression closely tied to CDK9 dysfunction
Researchers describe a newly observed role for the protein Cyclin Dependent Kinase 9 (CDK9) in regulating DNA repair during cellular division, where errors can become the origin of cancerous tumor growth. Through a process called phosphorylation, the experiment simulated the interaction of CDK9 with the other proteins and genes involved in cell division and cancerous tumor growth.
This study is based on the use of CRISPR/Cas9 technology to generate an experimental line of HeLa cervical carcinoma cells that no longer express the 55kDa molecular weight isoform (CDK9-55KO).
The study is published in Oncogene. The research group was led by Prof. Antonio Giordano, M.D., Ph.D., Director of the Sbarro Institute for Cancer Research and Molecular Medicine, Temple University, Professor of Pathology at the University of Siena, and Founder of the Sbarro Health Research Organization (SHRO).
CDK9, first discovered by Giordano in 1994, is a multifunctional protein kinase and its expression is strongly altered in tumors. The paper describes the role of the isoform CDK9-55kDa in DNA damage response, one of the main cellular mechanisms modulated for cancer therapy.
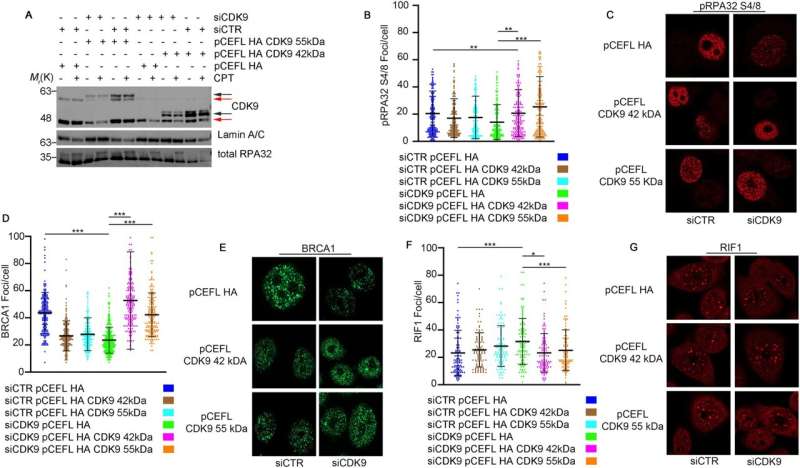
In the first analysis, the scientists led by corresponding author Dr. Luigi Alfano, a researcher at the National Cancer Institute of Naples Pascale Foundation, demonstrated how the lack of the CDK9-55 protein negatively impacted the repair mechanism of homologous recombination, the most important process to avoid the formation of mutations within the DNA sequence.
In particular, the researchers carried out an experiment using a phosphoproteomic screening to reveal protein substrates regulated by CDK9 and observe how it interacts with the protein Cell Division Cycle 23 (CDC23), a subunit of a multiprotein complex Anaphase Promoting Complex Cyclosome (APC/C), which is implicated in the protein degradation of many oncogenes and tumor suppressors, leading to cancer progression.
Furthermore, the researchers demonstrated how CDC23 is phosphorylated on Serine 588 by the CDK9 kinase, the major phosphorylated amino acid of the CDC23 protein found in many tumors.
"This discovery allows us to add an important new step to the understanding of how cells choose which repair mechanism to implement," says Alfano, "favoring the conservation of genetic information and reducing the onset of mutations predisposing to cancer."
"The role of CDK9 allows us to pave the way for a generation of new pharmacological inhibitors," says senior author Giordano, "both in monotherapy or in combination with other drugs already currently in use to enhance their anti-tumor effect."
More information: Luigi Alfano et al, CDK9-55 guides the anaphase-promoting complex/cyclosome (APC/C) in choosing the DNA repair pathway choice, Oncogene (2024). DOI: 10.1038/s41388-024-02982-w