This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Study discusses liquid biopsy as a game changer for early lung cancer detection
In 2020, 1.8 million people succumbed to lung cancer. The late-stage discovery of lung cancer is one of the glaring challenges faced by the medical community. While surgical biopsies continue to remain the gold standard for cancer diagnosis, the invasive nature of the technique can lead to serious health complications.
Finding alternate therapies that are less invasive and complement current diagnostic practices, leading to early-stage detection, is the need of the hour. Health professionals advise high-risk individuals to undergo routine lung cancer screening. Low-dose spiral computed tomography is a low-radiation CT scan that can detect abnormal growth in the lungs. However, in suspected cases, it could lead to overdiagnosis and overtreatment.
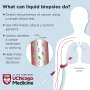
Liquid biopsy has recently emerged as a valuable tool for cancer diagnosis and treatment selection. The term "liquid biopsy" refers to a non-invasive test performed on blood or body fluids to detect cancer cells. It also detects circulating DNA, RNA, proteins, or other molecules released from tumor cells. In a recent review article, researchers from the U.S. and China highlight the recent progress in early lung cancer diagnosis using liquid biopsy.
The first author, Dr. Marina Bibikova from AnchorDx, Inc. in the U.S., comments, "Liquid biopsy, a test that can detect products derived from a tumor in body fluids, for example, in a simple blood draw, holds great promise as a non-invasive, easy, and accessible tool that can supplement or overcome the limitations of currently used methods for the early detection of lung cancer."
Their paper was published in Chinese Medical Journal Pulmonary and Critical Care Medicine.
Epigenetic changes and their markers are usually involved in cancer progression, of which DNA methylation signatures are noted in early cancer stages. Screening of DNA methylation profiles can, therefore, be a game changer in early lung cancer detection.
In 2017, the Epi proLung blood-based version of the lung cancer test, which used circulating DNA from plasma samples, could distinguish patients with lung cancer from those without cancer with 90% sensitivity and 73% specificity. AnchorDx's novel blood-based pulmonary diagnostic test, PulmoSeek from AnchorDx, can distinguish a malignant tissue from a benign tissue based on DNA methylation profiles with high sensitivity and reasonable accuracy.
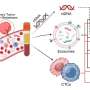
Some of these signatures or biomarkers are found in circulating tumor DNA (ctDNA) that are suspended freely in the blood. It is possible to track cancer progression, tumor size, and even treatment efficiency using ctDNA. Similarly, circulating tumor cells (CTCs) are potential biomarkers for early lung cancer diagnosis. CTCs are hostile cells that invade and metastasize healthy cells. These are present in extremely low quantities but, if detected, can provide insights into the recurrence and advancement of cancer.
MicoRNAs and exosomes are other possible indicators of lung cancer. Circulating antibodies that the body develops in response to tumor cells show early presence and can be detected as early as four years before lung cancer diagnosis. Additionally, proteins in the serum can be detected using ELISA and mass spectrometry.
Tumor cells convert healthy platelets into tumor-educated platelets, which work in favor of cancer cells and protect metastasis. These, too, can be detected using liquid biopsies.
In addition to being non-invasive, liquid biopsy has several other benefits, including low cost, genomic testing potential, and the ability to address the problem of tumor heterogeneity. It can also identify targetable mutations, track immunotherapy responses, and detect treatment resistance and relapse.
The Food and Drug Administration (FDA) has already approved liquid biopsy tools, such as Guardant360 CDx and FoundationOne Liquid CDx, for cancer therapy selection in clinical settings. The technique may soon become a standard diagnostic tool for lung cancer screening and detection.
While circulating biomarkers have diagnostic potential, they individually lack specificity or sensitivity. This can be resolved when current and novel diagnostic tools are used together. Furthermore, diagnostic tools should have the ability to detect high-risk, low-risk, and no-risk individuals, should be easy to implement in clinical settings, and should provide simple-to-interpret outcomes.
Dr. Bibikova says, "In addition to traditional biomarker tests, a multimodal diagnostic approach that combines multiple diagnostic methods—imaging, liquid biopsy, and clinical characteristics—can be used to improve the accuracy of diagnosis."
Indeed, a rigorous optimization of liquid biopsies could ensure their clinical utility in early lung cancer detection.
More information: Marina Bibikova et al, Liquid biopsy for early detection of lung cancer, Chinese Medical Journal Pulmonary and Critical Care Medicine (2023). DOI: 10.1016/j.pccm.2023.08.005