This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Genetic test identifies patients with triple negative breast cancer who are unlikely to respond to immunotherapies

Researchers have developed a genetic test that can identify how patients with triple negative early-stage breast cancer will respond to immunotherapy drugs. This means that patients who are unlikely to respond to these drugs can avoid the adverse side effects associated with them and can be treated with other therapies.
Professor Laura van 't Veer told the 14th European Breast Cancer Conference that the latest results from the I-SPY2 trial suggest that the current standard of care for patients with triple negative breast cancer should be reconsidered.
"Immunotherapy drugs can have very severe, irreversible adverse side effects, as observed in the I-SPY2 trial. The findings I'm presenting today should provoke a discussion about whether giving immunotherapy drugs to all patients with triple negative disease, which has recently become the standard of care in most countries, is the right strategy.
"Our research shows that it should be adapted so as to select only those patients who are very likely to benefit from this treatment. Patients who are unlikely to respond could then receive alternative therapies," said Prof. van 't Veer, who is Professor of Laboratory Medicine, Co-leader of the Breast Oncology Program and Director of Applied Genomics at the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, U.S.
The I-SPY2 trial was established in 2010 to find ways to screen new anti-cancer drugs and match them to specific biological markers in patients with breast cancer at high risk of early recurrence. Researchers developed an immune classifier, called ImPrint, composed of 53 genes, which can be used in the clinic to predict the likelihood of a patient responding to immunotherapies by looking at the biology of the patient's tumor. It classifies tissue from patient biopsies into "likely responder" or "likely non-responder" to immunotherapy.
Today, Prof. van 't Veer presented the results for an updated version of the classifier, ImPrintTN, refined to provide more accurate predictions for patients with triple negative breast cancer—the type of breast cancer in which the cancer cells are not fueled by estrogen, progesterone or the HER2 protein.
"Previously, we showed that gene expression signatures representing the active components of the immune system can predict the response to pembrolizumab—an immune oncology drug that targets PD1, which is a protein on the surfaces of cells that plays a role in the immune system. Both patients with triple negative disease and also patients with hormone receptor positive breast cancer, who had not yet received treatment and whose tumors had this active immune biology, showed a significant, up to three times higher, pathologic complete response rate when treated with pembrolizumab," she said.
A pathologic complete response (pCR) is when the cancer shrinks and even disappears after drug treatment.
She continued, "This classifier had a good performance across triple negative and hormone receptor positive breast cancers combined, and had very high positive predictive value for hormone receptor positive breast cancers, meaning that it identified those cancers that would likely respond to an immunotherapy."
"However, we noticed that the performance of ImPrint for triple negative breast cancers, where immune oncology drugs are now standard of care, was not yet good enough to identify patients in whom a 'likely response' to immunotherapy was so low that harm from serious side-effects would be higher than the benefit.
"This new work now presents an update of the ImPrint classifier specifically for triple negative breast cancers, ImPrintTN. We found that it can predict patients who are unlikely to respond to immunotherapy, so that the harms from the treatment are greater than the benefit. This means it would be acceptable for them to forgo an immunotherapy drug in order to avoid the risk of these sometimes life-long irreversible adverse effects."
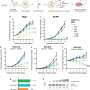
Out of 150 patients receiving immunotherapy in four arms of the trial and 128 patients in the control arm, receiving taxane and anthracycline chemotherapy, ImPrintTN identified 66% of patients with triple negative breast cancer as being likely to respond to immunotherapy.
Patients in the immunotherapy arms had been divided equally into two sets: a training and a test set, and the sets were also balanced so as to have an equal number of "responders" and "non-responders."
In the independent test set, pCR rates were 71% compared to 22% in patients identified as unlikely to respond. When all five arms were combined (the test and the training sets), pCR rates were 74% in patients identified as likely to respond and 16% in those that ImPrintTN classified as unlikely to respond. This improves on the previous version of ImPrint where pCR rates were 38% among patients that it identified as unlikely to respond.
In the control arm of the trial where patients had been treated with standard of care chemotherapies only, pCR rates were 30% among patients identified by ImPrint as "responders" and 15% among "non-responders."
"The likelihood of an immunotherapy drug response for triple negative cancers that are ImPrint-positive, remains very high at 74%, while among patients that ImPrintTN identified as likely 'non-responders' the pCR rates for immunotherapy are now very low at 16%—low enough for the harm from immunotherapy drugs to outbalance the benefit in these patients," said Prof. van 't Veer.
"This is a clinically important improvement and suggests that ImPrintTN may help to inform prioritization of immunotherapies versus other treatments for patients with triple negative breast cancer in order to best balance likely benefit versus the risk of serious and irreversible adverse effects. There is a subgroup of patients where the harm of these drugs outweighs the therapeutic benefit.
"Once ImPrintTN has been validated further, immunotherapy drugs should only be given to patients with triple negative or HR positive disease who have a high likelihood of benefiting."
Professor Michail Ignatiadis from the Institut Jules Bordet in Brussels, Belgium, is Chair of the 14th European Breast Cancer Conference and was not involved in the research. He said, "It is increasingly appreciated that the 'one size fits all' approach is not optimal for the systemic treatment of patients with early triple negative breast cancer. The identification of biomarkers to identify patients that do not need neoadjuvant immunotherapy is an unmet medical need. The results presented today show that ImPrintTN is a promising such biomarker that if further validated, can spare many women the short and long-term toxicity of these drugs."
More information: Abstract no: 2LBA, "Immune subtyping in the Response Predictive Subtypes (RPS) identifies a subset of triple negative (TN) early- stage breast cancer patients with a very low likelihood of response to neoadjuvant immunotherapy (IO): results from 5 IO arms of the I-SPY2 TRIAL", Wednesday 20 March, Young Investigator Innovation Award and oral abstract session.