This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Researchers demonstrate interaction between metabolic health and healthy aging
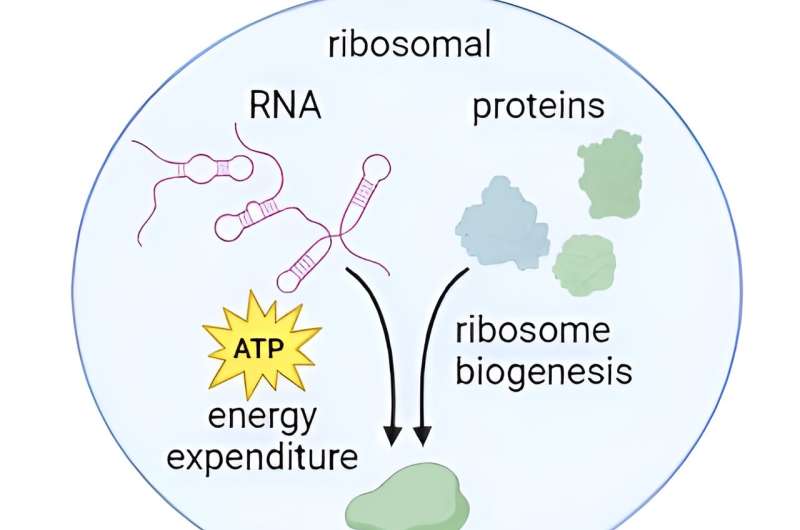
Ribosomes, the "translation factories" of the cell, are cellular organelles that play a central role in protein synthesis, a vital process for all living organisms. These tiny structures themselves consist of ribonucleic acid (RNA) and proteins and are essential for the survival and normal functioning of the cell, as the proteins they produce are required for nearly all cellular processes, including structure, function, and regulation.
The production of ribosomes begins when RNA polymerase I (Pol I) synthesizes pre-rRNA, a precursor transcript of the mature ribosomal RNA (rRNA) that later becomes part of the ribosome. Previously, it was shown that lower activity of Pol I is associated with longer life, but there was no precise explanation for this.
A study published in Nature Communications now demonstrates that reducing Pol I activity in the nematode Caenorhabditis elegans leads to improved mitochondrial function and a more stable metabolism, thereby extending lifespan. These findings also have implications for human aging.
The study brought together the expertise in organismal and cellular metabolism and aging of Maria Ermolaeva's research group, Stress Tolerance and Homeostasis, at the Leibniz Institute on Aging—Fritz Lipmann Institute (FLI) in Jena, and the experience in Pol I transcription and ribosome biogenesis of Holger Bierhoff's research group "Epigenetics of Aging" which is affiliated with the Friedrich Schiller University Jena and also associated with the FLI.
rRNA biosynthesis and ribosome biogenesis collectively consume up to 60% of the cellular energy. In this context, the research team's findings support the idea that Pol I inhibition extends lifespan, healthy lifespan, and metabolic fitness by actively lowering energy consumption.
Interestingly, reduction of Pol I activity not only generates an ATP surplus that can be used by cellular quality control pathways, but also elicits metabolic rewiring to curb triglyceride expenditure and mitochondrial respiration. Because mitochondrial respiration is a key source of reactive oxygen species (ROS) and mitochondrial damage during aging, the reduction of this activity confers an anti-aging effect.
The researchers also genetically modified C. elegans to increase Pol I activity. While these animals demonstrated early advantage in fitness showing increased body size and neuromuscular performance, their metabolism deteriorated more quickly, leading to premature aging and early death. Finally, it was shown that the life-extending capacity of reduced Pol I activity is not limited by aging and elicits benefits also in late life.
"It was very exciting for us to discover a longevity intervention that is effective in late life because previous work by us and others determined old age to be a limiting factor for the efficacy of commonly known interventions such as metformin and dietary restriction," says Maria Ermolaeva.
"It was also exciting to discover a geroprotective intervention that acts by actively suppressing energy metabolism. Studies in very long-lived species like bowhead whale and naked mole rat found reduced energy metabolism among their key genetic advantages. Now we uncovered an intervention that can potentially replicate this positive trait in humans."
Since the researchers also included human cells in their studies, the findings might be also relevant for human aging. "Aging is the major risk factor for developing cancer, which in turn is almost always associated with elevated ribosome biogenesis" says Holger Bierhoff. "A moderate reduction of rRNA synthesis, especially at old age, may be a viable option not only to improve metabolic health, but also to reduce the cancer risk," he adds.
Finally, the study highlights that excessive dietary protein intake—a common self-intervention that facilitates muscle growth in combination with strength exercise—bears potential risks, because it also stimulates Pol I activity. Akin to the findings in Pol I-enhanced nematodes, the overconsumption of protein-rich foods and supplements may improve physical fitness in the short-term, but may accelerate the metabolic aging process in the long-term, potentially impeding organ function in late life.
The study provides important insights into the molecular mechanisms underlying longevity. Targeted regulation of Pol I activity could potentially open up new avenues for the development of therapies to promote healthy aging.
More information: Samim Sharifi et al, Reducing the metabolic burden of rRNA synthesis promotes healthy longevity in Caenorhabditis elegans, Nature Communications (2024). DOI: 10.1038/s41467-024-46037-w