This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Metadata to ensure quality research and animal welfare
A study conducted by a public-private research team, including the University of Lausanne (UNIL), establishes the first minimal metadata set for animal experimentation, a crucial step towards maximizing the sharing and reproducibility of research data and limiting the use of animals. The study, published in LabAnimal, is a real call to action for the major stakeholders in biomedical research.
Biomedical research has entered a new era, with an explosion of data generated as a result of technological and digital advances. However, quantity does not necessarily guarantee scientific progress. To increase knowledge, data must be shared so that it can be re-interrogated in other contexts or reproduced by others.
Data reuse and reproducibility are particularly relevant to research involving animals in order to limit their use to a strict minimum and only when they enable new questions to be answered. Metadata is an essential part of this process. They contain descriptive and administrative information, as well as details on how the original data was collected.
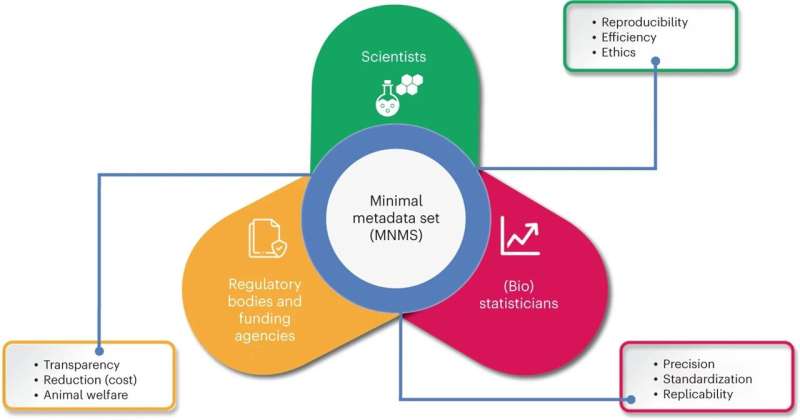
However, existing metadata for biomedical research on living animals lack a standard that could ensure the interoperability of the associated raw data. Defining the minimal metadata set (MNMS) required to describe the data generated by an experiment would enable the impact of such data to be extended to multiple research disciplines and thus limit the use of animals.
Leonardo Restivo, head of the Neuro-Behavioral Analysis Unit in the Department of Fundamental Neurosciences at the Faculty of Biology and Medicine of the University of Lausanne (UNIL) and co-author of the study, explains, "We embarked on this study following meetings and discussions that emerged within the framework of the European Cooperation in Science & Technology (COST-TEATIME action) because it has become urgent to act."
Fair and ethical data
The team behind the study proposes a minimal metadata set (MNMS) designed to enable in vivo data reuse.
"We didn't start from scratch. We aligned ourselves with a guideline for improving animal research reporting called ARRIVE 2.0 that existed since 2010. What's more, we wanted this metadata set to contribute to making data from living animals compliant with the FAIR data concept," explains Leonardo Restivo. The FAIR approach was developed in response to the accessibility of the internet and big data to make data Easy to Find, Accessible, Interoperable, and Reusable (FAIR).
The result is a table, a sort of checklist, presenting crucial aspects of animal experimentation, such as housing conditions, imaging, surgery, methods of compound administration, and standardized protocols, but also the age or genetic status of the animals, which must be documented in the metadata when collecting experimental data.
The study goes a step further, demonstrating that MNMS is applicable to biomedical research by showing a concrete experimental case carried out in a biopharmaceutical company.
Beyond the data
The authors also propose scenarios in which MNMS should be implemented. Various research environments and situations, such as the issue of data confidentiality, provide concrete possibilities and levers to meet the challenges of data reallocation at different levels.
"For the moment, it's a solid base proposed by our research team. The idea is for scientists, stakeholders, and decision-makers to take it up and develop it further," says Leonardo Restivo.
More information: Anastasios Moresis et al, A minimal metadata set (MNMS) to repurpose nonclinical in vivo data for biomedical research, Lab Animal (2024). DOI: 10.1038/s41684-024-01335-0