This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Revitalizing vision: Metabolome rejuvenation can slow retinal degeneration
Gene therapy may be the best hope for curing retinitis pigmentosa (RP), an inherited condition that usually leads to severe vision loss and blinds 1.5 million people worldwide.
But there's a huge obstacle: RP can be caused by mutations in over 80 different genes. To treat most RP patients with gene therapy, researchers would have to create a therapy for each gene—a nearly impractical task using current gene therapy strategies.
A more universal treatment may be forthcoming. Using CRISPR-based genome engineering, scientists at Columbia University Vagelos College of Physicians and Surgeons are designing a gene therapy with the potential to treat RP patients regardless of the underlying genetic defect.
The experimental therapy—which is still several years away from testing in people—corrects a metabolic error in the eye's light-sensing cells that is common to most forms of the disease.
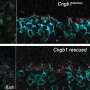
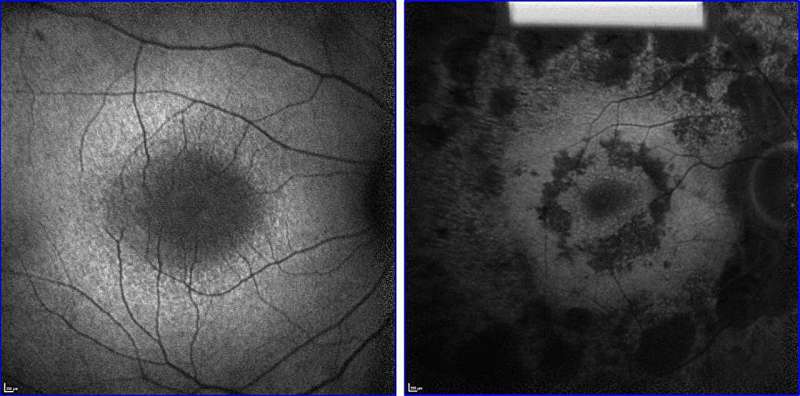
In a study published in Cell Reports Medicine, the genome engineers report that their CRISPR-based gene therapy can delay the progression of RP for about one month in mouse models of the disease, equivalent to about 10 years in humans.
The models included two genetically distinct types of RP, suggesting that the therapy would work for many forms of the disorder. In contrast, the lone FDA-approved RP gene therapy is suitable only for a few hundred patients in the United States whose condition is caused by the gene encoding the RPE65 isomerase.
"A universal precision metabolome rejuvenation would be a vast improvement over the limited options now available to most RP patients, which do little to prevent blindness," says study leader Stephen Tsang, MD, Ph.D., professor of ophthalmology and pathology & cell biology.
The clinical challenge
In recent years, researchers have hypothesized that although each of the nearly 80 RP genes initiates the disease in a different way, loss of vision usually occurs because the rods (the photoreceptors responsible for night vision) are progressively starved of energy.
Rods obtain energy by breaking down glucose to generate ATP via a process called glycolysis. A decrease in ATP energy production through glycolysis has been observed in both diseased and aging rods.
"The gradual loss of rods causes impaired night vision and loss of peripheral vision," says Nicholas Nolan, the study's first author and a doctoral student in biomedical engineering in Tsang's laboratory.
But because the rods' energy supply is connected to the energy supply of other retina cells, "once the rods start to starve and degenerate, that drives a wave of starvation and degeneration of the cones—the photoreceptors responsible for daylight color vision and visual acuity," Nolan says. "For patients, that corresponds to a devastating loss of day vision."
In many cases, RP leads to total vision loss.
Enhanced glycolysis rejuvenates photoreceptor neurons
Nolan and Tsang proposed that rejuvenating the glycolytic metabolome specifically within rods could prevent degeneration in both rods and cones, slow down the aging of the retina, and preserve vision. Although oral medications like daprodustat, which enhance glycolysis, have received FDA approval, they impact the entire body and may cause undesirable side effects.
"Our solution is to use CRISPR-based gene therapy to precisely rejuvenate the glycolytic metabolome in rod cells," Nolan explains.
Nolan and Tsang's experimental therapy is designed to use CRISPR gene therapy to edit a gene called PHD, thus triggering a cascade of changes that ultimately rejuvenate rod glycolysis.
To create the novel precision reprogramming, the researchers packaged the components of the CRISPR editing system into adeno-associated viral vectors, which when injected into the retina, expressed their genetic payload only in rod cells.
Because of the energy interconnections between rods and the rest of the retina, "by boosting rod glycolysis, we're making sure the rods are getting enough energy, but we're also keeping their support structures nourished. Overall, this increases the resilience of both rods and cones," Nolan says.
Next steps
The next step for the proposed gene therapy is to validate the mechanism of action with the lab's established rodent models of degeneration before translating these studies into a larger animal model with an eye structure that more closely resembles that of a human.
"These studies will provide a clearer understanding of the potential of precision rejuvenating therapy and will enable a more comprehensive safety assessment to be conducted."
"We hope to extend the benefits of our gene therapy even further as we learn more about rejuvenating glycolytic metabolome and how it's affected by RP and aging," Tsang says.
More information: Nicholas D. Nolan et al, CRISPR editing of anti-anemia drug target rescues independent preclinical models of retinitis pigmentosa, Cell Reports Medicine (2024). DOI: 10.1016/j.xcrm.2024.101459