This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers unlock secrets of birth defect origins, offering early detection and prevention strategies
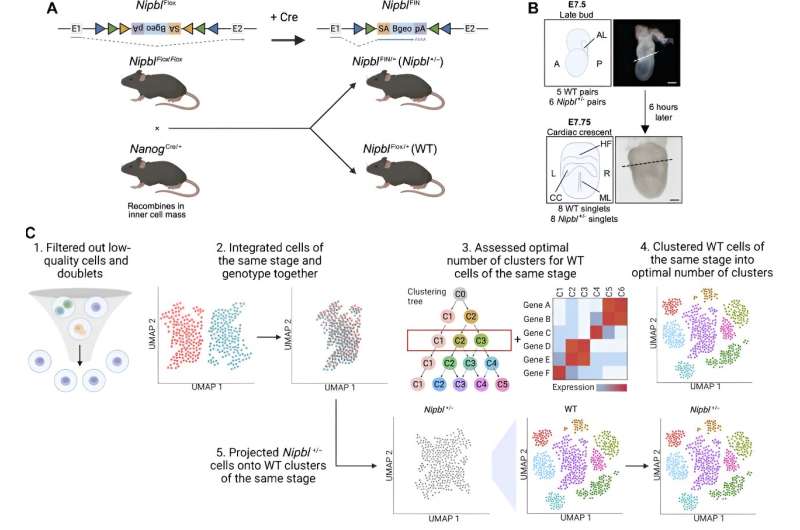
A new study led by the University of California, Irvine, has revealed a potential shift in our basic knowledge of the origins of birth defects, which affect about 3% of babies born in the United States each year. The findings offer new avenues of exploration for early detection and prevention strategies.
The work, published online recently in the journal Science Advances, identified crucial genetic interactions during the earliest stages of embryonic development that predicted birth defects characteristic of Cornelia de Lange syndrome and may offer clues about the genetic origins of many types of birth defects.
Researchers made a profound discovery relating to the gene Nipbl—a deficiency of which causes most cases of Cornelia de Lange syndrome—and its intricate interplay with another gene, Nanog.
"Our data suggest that Nipbl and Nanog are more than just necessary players in early embryonic development. They are critical regulators that, when misregulated together, have far-reaching impacts on embryogenesis and the development of many tissues and organs," said co-author Stephenson Chea, UCI developmental and cell biology graduate student researcher.
This pivotal venture began with a cutting-edge approach to mapping gene expression in early embryonic cells. By employing single-cell RNA sequencing and innovative bioinformatic techniques, the team charted the gene expression profile of every cell population present during gastrulation, a foundational stage of development.
The differences observed in gene expression and cell population sizes between normal embryos and those that were Nipbl-deficient provided unprecedented insights into the earliest origins of birth defects.
"This is the first time in this complex developmental syndrome that we've been able to uncover such profound gene expression differences at a vital developmental juncture, leading us to believe that we are beginning to understand a fundamental aspect of the way in which congenital birth defects originate during early development," said co-author Anne Calof, UCI professor of anatomy and neurobiology.
The implications reach far and wide. Pinpointing the stages in which the developmental pathways of different tissues diverge due to genetic anomalies introduces the possibility of intervening medically.
"Understanding this precise timing opens a window not just for diagnosis but, potentially, for identifying biomarkers that would be important for developing therapeutic interventions as well. This is where our research connects with real-world application," said team member Dr. Arthur Lander, UCI Donald Bren Professor and Distinguished Professor of developmental and cell biology.
Early detection of and intervention for birth defects can significantly improve life outcomes and reduce long-term health care costs, benefiting affected individuals, their families and society in general.
The study underscores a critical transition from observation to action in genetic research. The knowledge gained provides a framework for further research that could one day lead to preventive treatments, ensuring that children worldwide have a healthier start in life.
More information: Stephenson Chea et al, Gastrulation-stage gene expression in Nipbl +/− mouse embryos foreshadows the development of syndromic birth defects, Science Advances (2024). DOI: 10.1126/sciadv.adl4239