This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Team assesses biological functions, diseases and therapeutic targets of heat shock protein 90
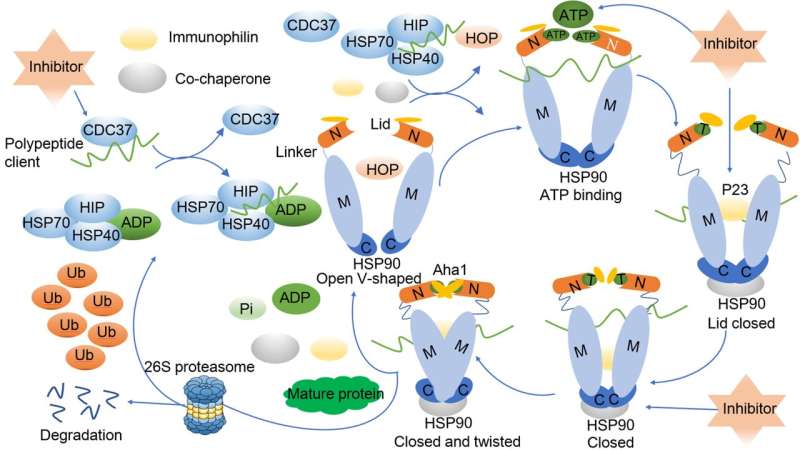
A review titled "Heat shock protein 90: biological functions, diseases, and therapeutic targets" and published in MedComm, conceived by Dr. Guiqin Hou, has been written by her graduate students Huiyun Wei and Yingying Zhang (the State Key Laboratory of Esophageal Cancer Prevention & Treatment, Key Laboratory of Advanced Drug Preparation Technologies, Ministry of Education, School of Pharmaceutical Sciences, Zhengzhou University).
Heat shock protein 90 (HSP90) stands out as a prominent member within the HSP family, holding a pivotal role in cellular protection and maintenance. Its functions encompass the facilitation of folding, stabilization, and modification of various protein substrates referred to as client proteins. Hsp90's significance in cellular activities cannot be overstated, as it ensures the proper functioning of client proteins, many of which are implicated in diseases like cancer, Alzheimer's, neurodegenerative disorders, and infectious ailments.
Given the crucial role of these client proteins across diverse pathologies, there is a burgeoning interest in targeting HSP90 and its co-chaperones for potential therapeutic interventions. Wei and Zhang present a comprehensive overview of the biological underpinnings of HSPs alongside the distinctive structural features specific to HSP90. Furthermore, they delve into the regulatory mechanisms governed by heat shock factor-1 (HSF-1) in modulating HSP90 activity, shedding light on the intricate dynamics of the chaperone cycle of HSP90.
Drawing upon a comprehensive grasp of HSP90's biological functions, Wei and Zhang offer a detailed synthesis of its pivotal role across various disease spectra, with a particular emphasis on its implications in cancer. Specifically, they delve into the intricate relationship between HSP90 and tumor resistance. Moreover, they meticulously examine the progress made in developing HSP90 inhibitors for cancer therapy, both in preclinical and clinical arenas, providing a nuanced analysis of their efficacy and limitations.
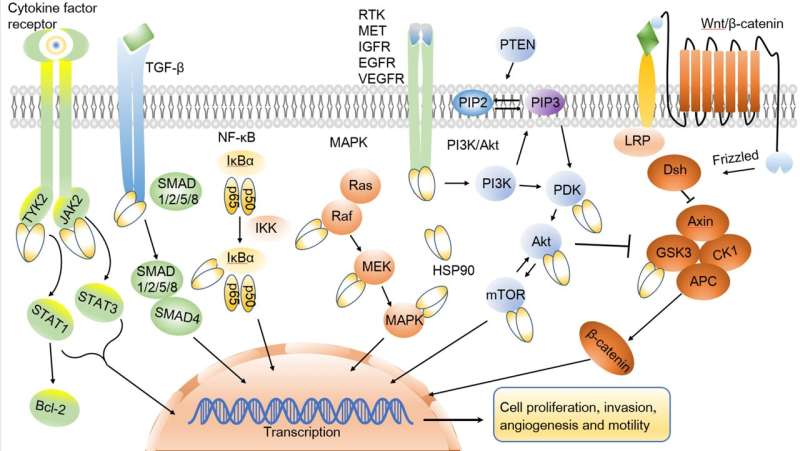
While mechanistic studies have showcased promising therapeutic effects of HSP90 inhibitors, the outcomes of clinical trials have been inconsistent. Presently, only a handful of HSP90 inhibitors, such as pimitespib approved in Japan for GIST treatment, have gained clinical approval.
Crucially, Wei and Zhang present fresh perspectives, advocating for a shift from focusing solely on "single parts" to considering "two or more parts" concurrently. This encompasses exploring isoform-selective inhibitors, conducting molecular dynamics studies, scrutinizing diverse cell death mechanisms, optimizing dosing regimens, harnessing cutting-edge technologies like cryo-electron microscopy (cryo-EM), and advancing efforts to uncover finer nuances of HSP90-client interactions. Such profound insights pave the way for the development of more precise inhibitors.
Building upon an extensive comprehension of HSP90, Wei and Zhang illuminate the simultaneous presence of challenges and opportunities in targeting HSP90 for cancer and disease management. This comprehensive review not only propels forward innovative therapeutic approaches but also fosters optimism for improved treatments spanning various disease landscapes.
More information: Huiyun Wei et al, Heat shock protein 90: biological functions, diseases, and therapeutic targets, MedComm (2024). DOI: 10.1002/mco2.470