This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers uncover how virus causes cancer, point to potential treatment
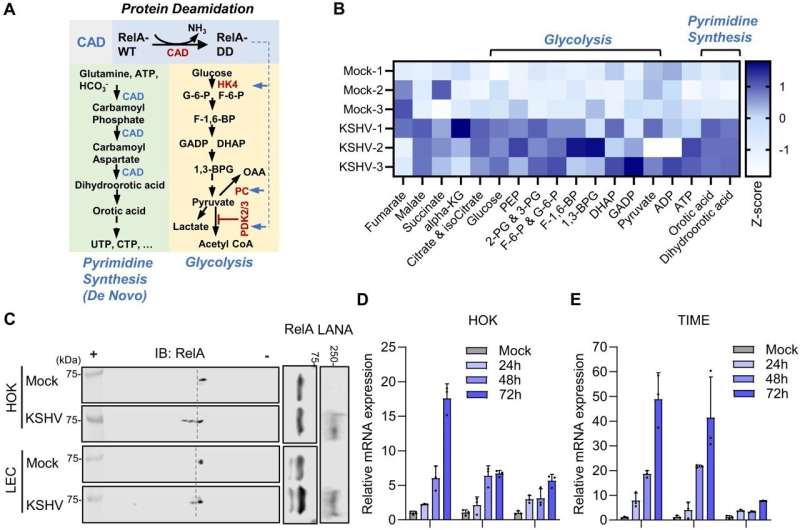
Cleveland Clinic researchers have discovered a key mechanism used by Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), to induce cancer. The research points to effective new treatment options for KSHV-associated cancers, including Kaposi's sarcoma, primary effusion lymphoma, and HHV8-associated multicentric Castleman disease.
"Our findings have significant implications: viruses cause between 10% to 20% of cancers worldwide, a number that is constantly increasing as new discoveries are made. Treating virus-induced cancers with standard cancer therapies can help shrink tumors that are already there, but it doesn't fix the underlying problem of the virus," said Jun Zhao, Ph.D., of Cleveland Clinic Florida Research & Innovation Center.
"Understanding how pathogens transform a healthy cell into a cancer cell uncovers exploitable vulnerabilities and allows us to make and repurpose existing drugs that can effectively treat virus-associated malignancies."
The Nature Communications study, led by Dr. Zhao, reveals that KSHV manipulates two human enzymes called CDK6 and CAD to reshape the way human cells produce new nucleotides—the building blocks of DNA and RNA—and process glucose. The changes to how infected cells grow and how KSHV persists put cells at a much higher risk of forming tumors and play a crucial role in causing cancer.
The team showed the virus activates a specific pathway driving cell metabolism and proliferation. Inhibiting this process with existing FDA-approved breast cancer drugs reduced KSHV replication, blocked lymphoma progression, and shrunk existing tumors in preclinical models.
Like other herpesviruses, KSHV often has no symptoms initially and remains in the body after primary infection. The virus stays dormant, suppressed by the immune system. However, KSHV can reactivate when immunity is weakened—as in older people, those with HIV/AIDS, and transplant recipients. In these high-risk groups, the now active virus can trigger aggressive cancers.
KSHV-induced cancers are fast-acting, aggressive, and difficult to treat. An estimated 10% of people in North America and Northern Europe have KSHV, but this ranges throughout the globe. More than 50% of individuals in parts of Northern Africa are estimated to have the virus. Experts estimate these rates are higher, as KSHV often goes undiagnosed because of a lack of symptoms. These findings have implications that reach past KSHV; researchers can apply knowledge about KSHV to other cancer-associated viruses that might use the same process to cause cancer.
Dr. Zhao collaborated with Michaela Gack, Ph.D., Scientific Director of the Florida Research & Innovation Center, to understand the cells' metabolic processes to uncover the virus's vulnerabilities.
Rapidly replicating cancer cells reprogram metabolism to fuel growth. Meanwhile, most viruses cannot produce energy or necessary molecules on their own, so they rely on human cells to do the work for them. The team found that the virus takes over the host protein CDK6 and CAD, causing the infected cells to produce extra metabolites, which allows faster replication of the virus and an uncontrolled proliferation of the cells.
The research team treated pre-clinical models with a CDK6-blocking drug, Palbociclib, an FDA-approved breast cancer medication, as well as a compound targeting CAD. They saw significant decreases in tumor size and increases in cancer survival rates: most tumors virtually disappeared after about a month of treatment, and the remaining tumors shrank by around 80%. Survival increased to 100% for selected lymphoma cell lines.
Dr. Zhao and his team are working to better understand the connections among KSHV, CDK6/CAD pathway, and cancer formation. With the knowledge they obtain, they plan to implement and refine their experimental drug combinations for clinical trials.
"Both viruses and cancers could hijack cellular metabolism for pathogenesis," said Dr. Zhao. "By investigating these metabolic rewiring mechanisms, we aim to find the Achilles' heel of cancer-causing viruses and non-viral cancers. I'm excited to see what the future of this work holds."
More information: Quanyuan Wan et al, Hijacking of nucleotide biosynthesis and deamidation-mediated glycolysis by an oncogenic herpesvirus, Nature Communications (2024). DOI: 10.1038/s41467-024-45852-5