This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
World-first trial of regenerative hearing drug is successfully completed
Researchers at UCL and UCLH have successfully completed the first trial of a therapy designed to restore hearing loss. The REGAIN trial, the results of which were published in Nature Communications, was the first study of a treatment aimed at restoring lost hearing, focusing on a drug with the technical name gamma-secretase inhibitor LY3056480.
The researchers found that while the therapy did not restore hearing across the group of adults with mild to moderate hearing loss, a deeper analysis of the data showed changes in various hearing tests in some patients, suggesting the drug has some activity in the inner ear.
These so-called efficacy signals call for further development of LY3056480—using the learnings from this trial.
Trial participants were aged between 18 and 80, hailed from the UK, Germany, and Greece, and had mild to moderate hearing loss. 15 patients took part in the phase 1 trial, which showed the treatment was safe and well tolerated, and 44 patients took part in the phase 2a trial designed to establish if the drug worked.
Participants received three injections of the drug into the ear through the eardrum. Their hearing was tested with a range of tests before and after receiving the drug.
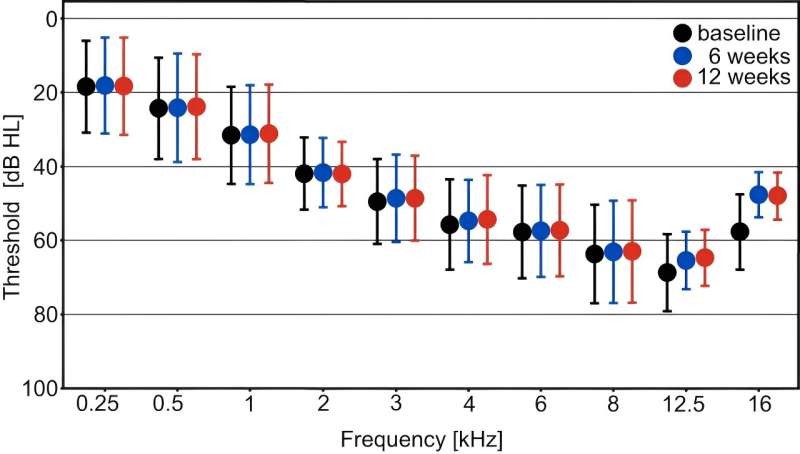
One test looked at the quietest possible sounds participants were able to hear. Another test assessed the ability to understand word sounds in a noisy background, which is the main unresolved problem for people with hearing loss.
45% of participants were able to identify some sounds that were at least 10 decibels quieter than they were previously able to hear at both 6 and 12 weeks after the start of treatment.
However, the research team set a higher bar to establish the impact of the drug—which was an average improvement of 10 decibels or more across three sound frequencies—and the hearing changes seen in the trial did not reach this more ambitious target.
Hearing loss is the most common sensory disorder in humans and an area of significant unmet need. It is primarily caused by progressive loss of inner ear sensory hair cells or their functionality. Presently, the only treatment is wearing hearing aids. These help people communicate but do not restore natural hearing or treat the underlying cause of hearing loss.
Because inner ear sensory hair cells do not naturally regenerate, hearing loss progresses with age.
Damage to these hair cells has long been considered irreversible, but various studies in animal models indicate that functioning inner ear sensory hair cells may be regenerated by a small molecule substance called a gamma-secretase inhibitor.
The REGAIN trial (Regeneration of inner ear hair cells with gamma-secretase inhibitors) was led by Professor Anne GM Schilder (UCL Ear Institute, UCLH Royal National ENT, Eastman Dental Hospitals, and NIHR UCLH Biomedical Research Centre). It was the first study of a regenerative hearing drug worldwide, delivered by an EU Consortium with clinical partners in Germany and Greece and the company Audion Therapeutics.
Professor Schilder said, "There are many important lessons from this study which will guide future studies of its kind. For example, the study will help how we best select the patients that may benefit from these new and highly targeted hearing treatments."
"This requires a better understanding of the mechanisms behind inner ear hearing loss and better hearing tests to identify its causes in patients. Big data and AI may speed up this process."
To progress this work, the UCLH BRC team, together with other BRCs, have set up the NIHR Hearing Health Informatics Collaboration (HIC) that will bring anonymized hearing data together from NHS hospitals across the UK for analysis with advanced computational techniques.
And locally, the team have opened a new patient registry called HEDGE, which offers people with hearing loss the opportunity to participate in research to help uncover the genetic and environmental factors, molecular pathways and mechanisms that cause hearing loss.
The huge demand among patients to take part in research was revealed by REGAIN.
Professor Schilder said, "We were contacted by more than 5,000 patients with hearing loss worldwide requesting to take part, illustrating the huge unmet clinical need."
UCL Ear Institute researchers were among the first to understand the role of a protein found in inner ear hair cells, which paved the way for new hearing therapies such as the one trialed in REGAIN.
More information: Anne G. M. Schilder et al, A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss, Nature Communications (2024). DOI: 10.1038/s41467-024-45784-0