This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Innovative microscopy demystifies metabolism of Alzheimer's
Alzheimer's disease causes significant problems with memory, thinking and behavior and is the most common form of dementia, affecting more than 50 million people around the world each year. This number is expected to triple by the year 2050.
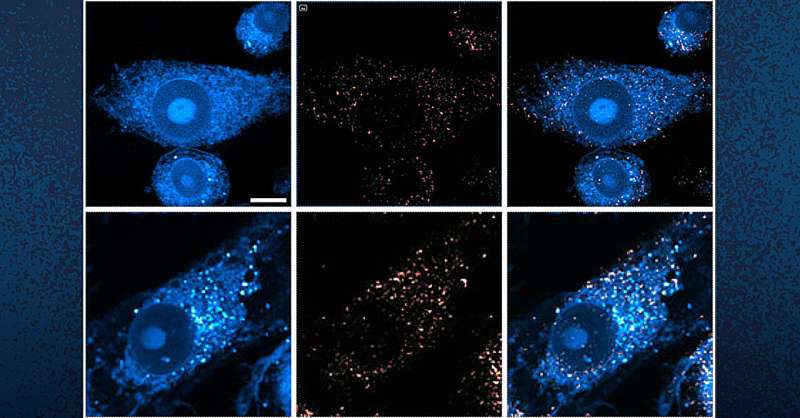
Using their own state-of-the art imaging technologies, scientists at the University of California San Diego have now revealed how the metabolism of lipids, a class of molecule that includes fats, oils and many hormones, is changed in Alzheimer's disease. They also revealed a new strategy to target this metabolic system with new and existing drugs. The findings are published in Cell Metabolism.
"Lipids have been associated with Alzheimer's for as long as we've known about the disease," said senior and co-corresponding author Xu Chen, Ph.D., an assistant professor in the Department of Neurosciences at UC San Diego School of Medicine, referring to the original 1907 report by Alois Alzheimer that described the unusual presence of fat deposits in the brain of the first person to be diagnosed with the disease.
"So much of the emphasis since then has been placed on tau and other proteins that the research community has, until the last decade or so, largely overlooked this important aspect of the disease."
"Driven by a keen interest in lipid droplet functions in aging and disease, we initiated this fruitful collaboration to harness cutting-edge SRS technology for studying lipid metabolism in tauopathy mouse brains," said Yajuan Li, M.D., Ph.D., a postdoctoral researcher in the Shu Chien-Gene Lay Department of Bioengineering at UC San Diego Jacobs School of Engineering. SRS imaging is an approach that analyzes the way molecules in a sample interact with laser light.
In the brain, lipids come in the form of tiny droplets that control a variety of processes, such as energy storage and cellular responses to stress. These processes are tightly regulated in typical brains, but in Alzheimer's or similar diseases, lipid droplet metabolism can malfunction. While scientists understand that there is a relationship between Alzheimer's and lipid metabolism, exactly how they influence one another has remained a mystery.
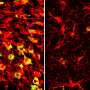
To answer this question, the team looked directly at lipid droplets in the brains of mice with excess tau protein. They used a state-of-the-art SRS imaging platform developed in Lingyan Shi's lab at the Jacobs School of Engineering. The platform makes it possible to take microscopic images of lipid droplets within cells without the use chemical dyes, which can alter the delicate molecules and compromise the results.
"Intriguingly, the inert lipid droplets observed in tauopathy brains exhibit similar behavior to those found in aging brains," said co-corresponding author Lingyan Shi, Ph.D., assistant professor of bioengineering at the Jacobs School.
"We are now focusing on understanding the underlying mechanisms by combing SRS imaging with other utilizing multidisciplinary techniques. Our approach is biologically neutral, so we're able to observe what's happening in the brain at the molecular level with as little interference as possible."
Shi and her team, including Li, pioneered the new approach, which uses a specially modified version of water, called heavy water, as a metabolic probe.
"Instead of using a typical chemical dye to stain lipids, we use heavy water that is naturally participating in the metabolic activities we're interested in," added Shi.
"This gives us a much clearer picture of how lipids are formed spatiotemporally, which would not be possible with other approaches. Our current focus is on comprehending the underlying mechanisms of these dynamic changes of lipid metabolism in the context of aging and diseases."
The researchers discovered that in brains with tauopathy, neurons accumulate excess lipids as a result of stress or damage. This influx forces neurons to offload the excess to immune cells in the brain, called microglia. These microglia then mount an inflammatory response that causes further stress to neurons, triggering a repeating and worsening cycle.
In addition to characterizing this process, they were also able to identify a critical enzyme, called adenosine monophosphate-activated protein kinase (AMPK) that orchestrates the cycle. According to the researchers, breaking this cycle could unlock new treatment options for Alzheimer's disease. Chen is particularly optimistic about the possibility of repurposing existing drugs that modify lipid metabolism.
"We don't think this is an incidental phenomenon," said Chen. "The evidence suggests that lipid metabolism is a driving mechanism for Alzheimer's disease. There are many drugs that target lipid metabolism in other body systems, such as in the liver, so we might be able to change this system quite dramatically using tools we already have."
More information: Yajuan Li et al, Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK, Cell Metabolism (2024). DOI: 10.1016/j.cmet.2024.03.014