This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
Bimekizumab yields meaningful response in hidradenitis suppurativa
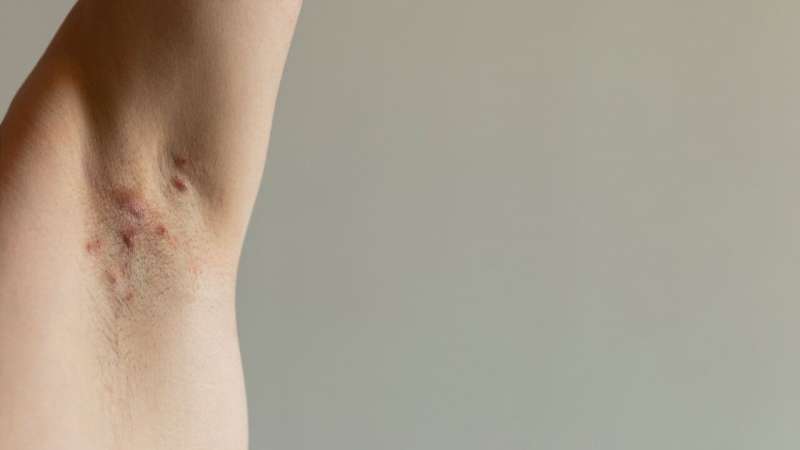
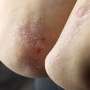
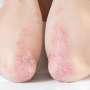
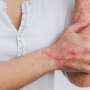
Bimekizumab is well tolerated and produces clinically meaningful responses in patients with hidradenitis suppurativa, according to a study published online May 22 in The Lancet.
Alexa B. Kimball, M.D., from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston, and colleagues examined the efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa in two identically designed, 48-week randomized, placebo-controlled trials (BE HEARD I and II [505 and 509 patients, respectively]).
Patients (aged 18 years and older) were randomly assigned to receive subcutaneous bimekizumab 320 mg every two weeks; bimekizumab 320 mg every two weeks to week 16, then every four weeks to week 48; bimekizumab 320 mg every four weeks to week 48; or placebo to week 16, then bimekizumab 320 mg every two weeks in a 2:2:2:1 ratio. The primary outcome was a hidradenitis suppurativa clinical response of at least 50% (HiSCR50) at week 16.
The researchers found that using modified nonresponder imputation, the primary outcome at week 16 was met in the group who received bimekizumab every two weeks; in both trials, higher responder rates were seen with bimekizumab versus placebo (odds ratios, 2.23 and 2.29, respectively, in BE HEARD I and II). HiSCR50 was also met in the group administered bimekizumab every four weeks in BE HEARD II (odds ratio, 2.42). The investigators observed a maintenance or increase in responses to week 48. In 8 and 5% of patients in BE HEARD I and BE HEARD II, respectively, serious treatment-emergent adverse events were reported.
"These data support the use of bimekizumab as a promising new therapeutic option for patients with moderate-to-severe hidradenitis suppurativa," the authors write.
More information: Alexa B Kimball et al, Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials, The Lancet (2024). DOI: 10.1016/S0140-6736(24)00101-6
Elisa Molinelli et al, Bimekizumab: dual inhibition as a promising tool in the management of hidradenitis suppurativa, The Lancet (2024). DOI: 10.1016/S0140-6736(24)00591-9
2024 HealthDay. All rights reserved.