This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
Illegal ecstasy takes step toward becoming legal drug for PTSD
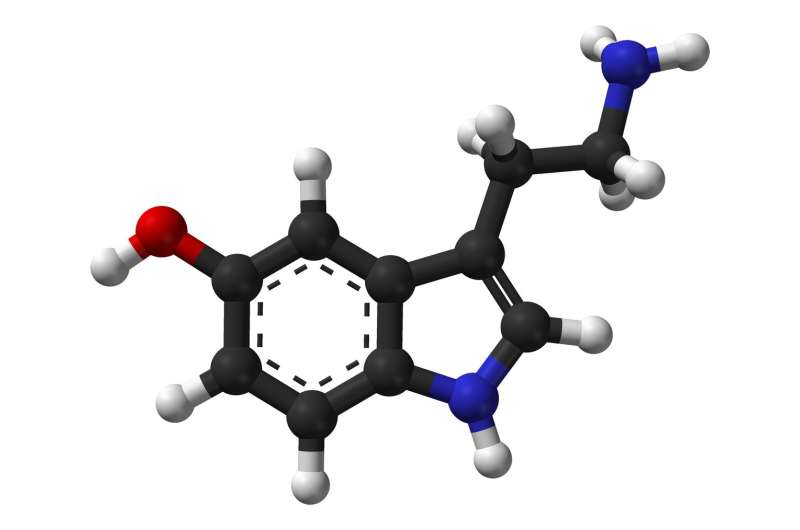
The first new PTSD drug in over 20 years is up for approval. It will require U.S. regulators to do something they've never done before: greenlight the mind-altering—and illegal—party drug known as ecstasy.
The new drug is a version of MDMA, also known as ecstasy, made by Lykos Therapeutics Inc., an unusual drugmaker started by a nonprofit that has for decades advocated to bring psychedelic therapy to the masses.
On June 4, the public will gain the first hint of how regulators are leaning when outside experts on the Food and Drug Administration's Psychopharmacologic Drugs Advisory Committee debate the data during a daylong session where both the company and FDA officials are expected to make their case. A decision on whether to approve the drug is likely by August.
"Everything about this is unprecedented," said Jonathan Alpert, chair of the council on research at the American Psychiatric Association.
Lykos, which is privately held, has proposed its pill be given in conjunction with 42 hours of talk therapy with two therapists, including three day-long sessions involving MDMA.
"We're on the precipice of integrating psychedelic medicine into mainstream medicine," Lykos Chairman Jeff George said speaking at a recent psychedelics conference in New York. Some 13 million Americans have PTSD.
Upping the ante, one influential government organization is already gearing up to provide the drug should it be approved. The Department of Veterans Affairs, which runs the country's largest health system and has an outsized PTSD patient population, says psychedelics have "significant potential." PTSD is a psychiatric disorder experienced after a traumatic event, like combat.
The FDA faces a dilemma. Controversy over Lykos' trial has exploded in recent weeks after a prominent group that assesses new treatments cited "substantial concerns about the validity of the results."
In a 44-page report released in March, the Institute for Clinical and Economic Review concluded that the evidence was "insufficient" to determine whether MDMA-assisted therapy has value. At a public meeting held by ICER, debate over whether benefit outweighed risk for Lykos' treatment was sometimes heated. In a vote, ICER advisers decided nearly unanimously that Lykos had not proven that the treatment was beneficial.
Chief among ICER's concerns was whether the trial could adequately prove MDMA worked because people on the therapy knew whether they had been given the psychedelic drug. The gold standard in research is what's known as a "double-blind" trial, meaning neither patient nor provider know who's on an experimental drug or a placebo in order to truly evaluate the difference. That wasn't the only problem.
"If you wanted to develop a study that would have every fatal flaw, you couldn't do better than what was done for MDMA," said Allen Frances, former chair of the psychiatry department at Duke University, who calls psychedelics the latest in a long series of overhyped psychiatry fads. The drug "is being rushed way too soon to commercial markets."
A need for new treatments
New treatments are badly needed for PTSD. There are just two drugs approved to treat it, and current therapies don't work for many people. In recent years, MDMA and other psychedelic drugs like magic mushrooms have been pitched as a panacea, for PTSD and a host of disorders including depression, anxiety, nicotine addiction and anorexia.
At least on paper, the results of the two phase three trials of MDMA are impressive: The treatment slashed symptoms so much that two-thirds of people no longer met the criteria for a PTSD diagnosis two months later. The drug was granted FDA breakthrough status in 2017, and was then granted priority review this year, which means the agency plans to move quickly to consider the drug for approval.
The results are "clearly better than what we have seen for the antidepressants currently approved by the FDA for PTSD," said Yale University psychiatrist John Krystal, who was not involved in the studies.
No one is quite sure exactly how MDMA helps people with PTSD or other disorders. It can cause an increased sense of well-being and social openness while altering a person's visual perceptions. Taking MDMA floods the brain with serotonin, a key mood-boosting chemical.
Some psychiatrists experimented with it as a communication enhancer during therapy sessions before it was banned by the Drug Enforcement Administration in 1985. The science suggests the drug may help the success of therapy by blunting the emotional response to traumatic memories and making it easier for people to talk through them.
But the same thing that may make the drug so powerful also makes its effectiveness difficult to evaluate. "When people know what they are receiving," Krystal said, "it can invalidate the value of placebo."
The ICER report also cited allegations that some negative patient experiences may not have been properly reported. It also said participants may have felt "pressured" to report good outcomes and suppress bad ones. The report also acknowledged an allegation of sexual misconduct during an early trial, including intimate contact between a patient and her therapists during a videotaped MDMA session, followed by alleged non-consensual sex with one of the therapists after all the sessions had been completed.
The ICER report was followed by a separate petition sent to the FDA commissioner signed over 70 independent researchers calling for an extended public hearing to address numerous issues with the way the trials were conducted.
Lykos said that the FDA okayed the design of the trials and that numerous steps were taken to minimize bias. The trials "were very rigorous; they were very well designed," said Amy Emerson, Lykos's chief executive officer. The company said it had third-party raters assess patient symptoms in order to minimize any influence from unblinding. She said the company carefully trained all the trial sites on proper reporting of adverse events. The company also said it investigated the abuse allegations and developed "policies and practices aimed to prevent, reasonably detect, and thoroughly respond to allegations of misconduct."
The first of many hurdles
The nonprofit behind Lykos, the Multidisciplinary Association for Psychedelic Studies, or MAPS, has been involved in almost all the major studies of MDMA for PTSD. That's contributed to allegations that its tight-knit culture has created a quasi-religious atmosphere where negative reports about the drug were discouraged or minimized.
In 2014, MAPS founded a for-profit public benefit company to conduct MDMA clinical trials. Earlier this year, the company changed its name to Lykos. MAPS remains the largest single shareholder. MAPS founder Rick Doblin, who's been the public face of medicalizing MDMA therapy, maintains the data is reliable.
"We did the very best we could do" to deal with the unusual challenges of studying psychedelic drugs, he said.
Cristina Pearse, a 51-year-old Boulder, Colorado, resident and child sexual abuse survivor who participated in the second phase 3 trial of MDMA in 2022 described her own experience as "miraculous." She suffered from persistent depression, anxiety and suicidal thoughts for decades before being diagnosed with PTSD. Within one hour of the first MDMA dose, she started to feel better. "It undid 47 years of trauma in that first session," she said.
At the ICER meeting, Meaghan Buisson, the patient who experienced the alleged abuse, cautioned against rolling the therapy out broadly. "There is no question on whether what happened to me will happen again to someone else. It is only a matter of time," she said during the public comment period of the meeting.
"That doesn't negate the lived experience of those who say they may have benefited from this trial, this simply means they dodged a bullet."
Jesse Gould, a former Army ranger and founder of a non-profit that helps vets with PTSD, the Heroic Hearts Project, testified to the desperate need for a new treatment. "If not MDMA, there's nothing out there in the pipeline," he said. "Do these risks that are outlined in the MDMA report really outweigh the enormous potential benefit?"
ICER's outside advisers agreed that there was an urgent need for new treatments. But in a warning sign for Lykos, the advisers voted nearly unanimously that the company had failed to prove that the treatment was beneficial overall. None of the advisers thought the treatment has been shown to be any better than standard talk therapy.
Even if Lykos' MDMA gets the green light from regulators, it will only be the first of many hurdles. The DEA will have to move MDMA to a less restrictive category of controlled substances. Administering it will require special training for therapists. And the many hours of prescribed therapy mean it could cost more than $12,000 per patient, according to ICER, which may make insurers hesitant to cover it.
2024 Bloomberg L.P. Distributed by Tribune Content Agency, LLC.