This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers establish microfluidic control technology for blood testing devices
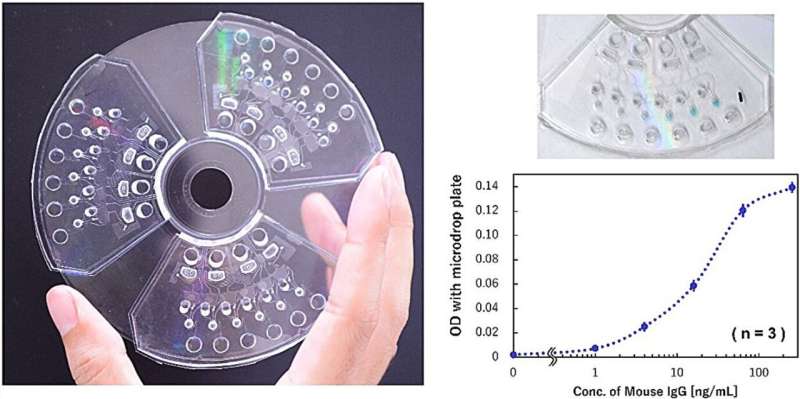
A research group in Japan has established a microfluidic control technology that can be applied to small blood testing devices and consequently developed a novel integrated immunoassay device. This microfluidic control technology is expected to address cost issues—one of the limitations to the social implementation of microfluidic chips—and realize a convenient blood testing device.
One team is supervised by Assistant Professor Shunya Okamoto of the Department of Mechanical Engineering, Toyohashi University of Technology, and another is supervised by Associate Professor Yoshiaki Ukita of the Graduate Faculty of Interdisciplinary Research, Faculty of Engineering, University of Yamanashi.
The results of this study were published in RSC Advances on April 26.
Compared with conventional analysis systems, those using microfluidic chips offer advantages such as minimized sample volume and shortened analysis time. However, a technology that precisely controls fluids in extremely minute spaces of approximately 50–300 μm is required.
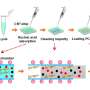
Enzyme-linked immunosorbent assay (ELISA) is an analysis technique used in blood analysis for measuring cancer biomarkers; however, although highly sensitive, ELISA requires complex fluid control.
Conventional studies have employed complex structures and operations, such as placing numerous valve structures and connecting pumps, to perform such fluid control, thus increasing the cost of devices and test chips; moreover, extensive user training is required to operate such devices. These issues have become major limitations to the social implementation of ELISA.
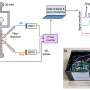
To address these issues, the researchers developed a centrifugal microfluidic chip with autonomous control functions. The chip comprises a microfluidic chip with only a simple channel structure that can be molded using mass-producible technologies such as injection molding. The team also performed analysis by simply rotating the chip at a steady rotation speed for several tens of minutes (steady rotation).
Thus far, they have demonstrated that this autonomous-controlled centrifugal microfluidic chip can perform ELISA, which requires complex fluid control. However, the main limitations are that numerous samples are required for analysis, and users require multiple chips and extensive dispensing operations to quantify the test substance.
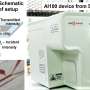
To address these limitations, the researchers developed a dispensing mechanism that can automatically distribute reagents instead of manual labor and demonstrated the operation of a "multi-sample microvolume simultaneous analysis device" that implements the dispensing mechanism in an analysis unit.
The analysis demonstration conducted using IgG antibodies as test substances showed that although the sample volume was sufficiently small—approximately 5 μL (mm3), which can be collected even by finger prick—and has the same detection sensitivity (63.4 pg/mL) as that of the previous manual analysis, the analysis time can be shortened by one-third to approximately 30 min.
These research results use prototype devices constructable at the laboratory level. However, in the future, the team will investigate practical applications by optimizing the design using mass-producible processing methods and materials.
This fluid control technology makes it possible to realize control with microfluidic chips with a simple structure. In addition, the centrifugal control device is also simple to operate because fluid control is feasible at a steady rotation speed. Therefore, this technology can contribute toward addressing cost issues—one of the limitations of the social implementation of microfluidic chips.
Currently, blood analyzers are installed only in central laboratories of general hospitals. However, with advances in cost reduction and device miniaturization, along with improved operability, blood analyzers can be installed in clinics, drugstores, and households, thus rendering blood tests accessible and improving people's quality of life.
More information: Shunya Okamoto et al, Automatic microdispenser-integrated multiplex enzyme-linked immunosorbent assay device with autonomously driven centrifugal microfluidic system, RSC Advances (2024). DOI: 10.1039/D4RA02656J