This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Investigators propose modified criteria for identifying anaphylaxis in infants and young children
A Mass General team has developed modified criteria that may help health care professionals more accurately identify anaphylaxis, a potentially life-threatening allergic reaction, in infants and young children.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, researchers cited an urgent need for training clinicians to recognize and manage anaphylaxis in this young patient population that is often difficult to diagnose.
"Infants and toddlers under three can be a challenge to evaluate, in part because of their limited ability to communicate symptoms," says senior author Michael Pistiner, MD, MMSc, Director of Food Allergy Advocacy, Education and Prevention at Mass General for Children.
"We're proposing modifications to current anaphylaxis criteria that consider age-specific symptoms and signs that are often overlooked or under-recognized. We believe our work will lead to more widespread recognition of a condition which is a true medical emergency."
Anaphylaxis is a serious systemic allergic reaction with a rapid onset that can result in death if not quickly treated. Among its common triggers are bee stings, peanuts, shellfish, and some medications.
Over the last few decades, increasing rates of anaphylaxis have been reported worldwide, particularly in infants. In the U.S., hospitalizations among children from food-induced anaphylaxis have been on the rise.
In 2006, the National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network (NIAID-FAAN) proposed and adopted criteria designed to help clinicians identify patients likely to be experiencing anaphylaxis.
In 2020, the World Allergy Organization (WAO) proposed modifications, though neither organization's criteria have been validated in infants and toddlers less than 2 years of age.
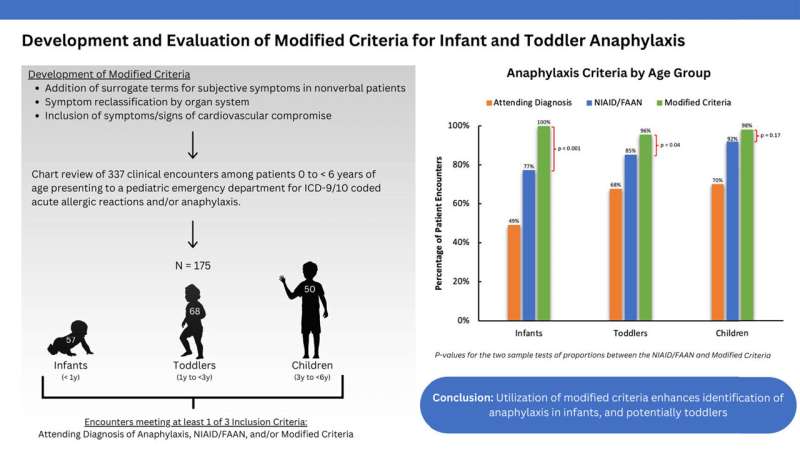
Mass General researchers set out to fill the gaps they perceived in those guidelines by identifying signs and symptoms that more precisely capture anaphylaxis in young children and incorporating them into a modified criteria.
In addition to commonly recognized symptoms like hives, swelling and vomiting their modifications include less recognized signs such as lethargy, inconsolability, coughing, diarrhea, cyanosis and rapid heart rate with no other explanation.
Based on a review of 337 cases of infants (less than 12 months), toddlers (12 to 36 months) and children (greater than 36 months) treated for suspected allergic or anaphylactic reactions in the pediatric emergency department of Mass General for Children, they evaluated their updated criteria against the current clinical standard.
They found that the NIAID/FAAN criteria identified 85% of all allergic/anaphylaxis encounters in the pediatric emergency department, while their expanded criteria captured 98% of such encounters.
Compared to existing standards, the modified criteria improved identification performance nearly 23% among infants, and more than 10% among toddlers.
"Some of our modifications are designed to accommodate the inability of infants to verbally express such symptoms as 'my tummy hurts,' 'my tongue is itchy,' or 'I'm dizzy,'" explains Anna Handorf, MD, a pediatrics specialist at Mass General for Children and co-first author.
"For these cases, we've added surrogate signs such as infants pulling their legs up to their chest, and crying as a proxy for abdominal pain."
The team also emphasized the need for enhanced training nationally of health care professionals—particularly clinicians without extensive pediatric experience—to recognize the unique signs of anaphylaxis presentation in infants and toddlers.
"We believe our modified criteria can greatly decrease the number of infants and toddlers whose anaphylaxis goes unrecognized," says Pistiner. "Future research will still be needed, including prospective, multicenter studies, to validate our findings."
More information: Anna Handorf et al, Development and Evaluation of Modified Criteria for Infant and Toddler Anaphylaxis, The Journal of Allergy and Clinical Immunology: In Practice (2024). DOI: 10.1016/j.jaip.2024.05.018





















