This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves Capvaxive pneumococcal 21-valent conjugate vaccine
The U.S. Food and Drug Administration has approved Merck's Capvaxive pneumococcal 21-valent conjugate vaccine for the prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults.
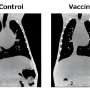
Capvaxive is specifically designed to help protect adults against the serotypes that cause the majority of invasive pneumococcal disease cases, including Streptococcus pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B, in individuals ages 18 year and older, and for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B in individuals 18 years and older.
The priority review was based on the pivotal Phase III STRIDE-3 trial, which compared Capvaxive to pneumococcal 20-valent conjugate vaccine in adults ages 18 years and older, who had not previously received a pneumococcal vaccine, as well as the Phase III STRIDE-5 and STRIDE-6 trials, which evaluated Capvaxive in vaccine-naive and vaccine-experienced adults.
Capvaxive includes eight unique serotypes not covered by other currently approved pneumococcal vaccines, but that are responsible for approximately 27 percent of invasive pneumococcal disease cases in adults ages 50 years and older and approximately 30 percent of cases in adults ages 65 years and older, according to epidemiological data.
The company says the vaccine covers serotypes responsible for approximately 84 percent of invasive pneumococcal disease in adults ages 50 years and older.
"Today's approval is a testament to our population-specific strategy behind Capvaxive, which demonstrated robust immunogenicity in a range of adult populations and is driven by a deep understanding of pneumococcal disease," Dean Y. Li, M.D., Ph.D., from Merck Research Laboratories, said in a statement.
"We are proud to provide Capvaxive as a new option specifically designed to help protect against the majority of invasive pneumococcal disease-causing serotypes in adults."
More information: More Information
Copyright © 2024 HealthDay. All rights reserved.