This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study suggests promising gene therapy for FOXG1 syndrome
A viral gene therapy developed by University at Buffalo researchers has reversed some brain abnormalities in infant mice with FOXG1 syndrome, a significant step toward one day treating children with this severe neurodevelopmental disorder.
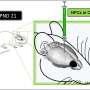
This mediated delivery of the FOXG1 gene via adeno-associated virus 9 (AAV9) is detailed in a study published June 5 in Molecular Therapy Methods & Clinical Development. A postnatal injection of the therapy in day-old mice rescued a wide range of abnormalities, the study found, including in parts of the brain responsible for language, memory and social interaction.
"Our findings highlight the efficacy of AAV9-based gene therapy as a viable treatment strategy for FOXG1 syndrome and potentially other neurodevelopmental disorders with similar brain malformations," says Soo-Kyung Lee, Ph.D., Empire Innovation Professor and Om P. Bahl Endowed Professor in the UB Department of Biological Sciences, College of Arts and Sciences, who led the study along with her husband, Jae Lee, Ph.D., professor in the department.
"This research asserts the therapeutic relevance of our approach in postnatal stages, which is a critical time frame for intervention."
The Lees' teenage daughter, Yuna, was diagnosed with FOXG1 syndrome at the age of 2. The researchers have since established themselves as leading experts on the disorder and are the principal investigators of UB's FOXG1 Research Center (FRC). The center, which launched earlier this year, as well as this recent study, are supported by the FOXG1 Research Foundation.
The study was co-led by Kathrin Meyer, Ph.D., principal investigator at Nationwide Children's Hospital in Columbus, Ohio. Other contributions represent the University of Pennsylvania and Samsung Medical Center in Seoul, South Korea.
Reversing structural abnormalities
A master regulator gene, FOXG1 is one of the most important genes for early brain development and its impairment can result in profound brain structure abnormalities.
The Lees previously established that the FOXG1 gene and protein remain active in mice after birth, so they wondered if restoring FOXG1 levels could reverse some of the abnormalities associated with FOXG1 syndrome.
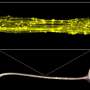
These abnormalities include failure to fully develop the corpus callosum, the bundle of nerves that connect the brain's two hemispheres and help integrate sensory and motor information with social interaction, executive function and language.
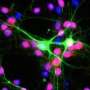
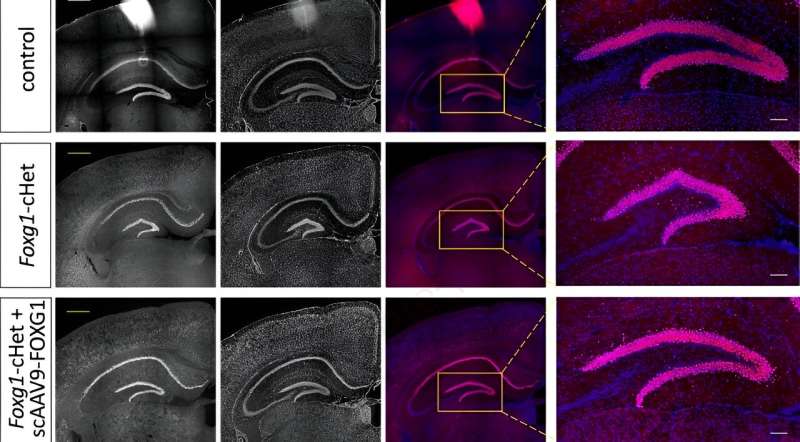
It's thought that correcting the corpus callosum postnatally would be extremely difficult given that it develops before birth, but, when injected into mice postnatally, the Lee team's viral gene therapy reconnected the callosal axons and restored the callosal nerves, substantially recovering the corpus callosum.
The therapy also increased the size of the dentate gyrus, the primary gateway for input formation into the rest of the hippocampus that is crucial for memory. This is one of only a few areas of the brain that continues to produce new neurons as mammals age into adulthood, making it a crucial target for postnatal treatments.

In addition, the therapy rescued areas of the brain related to signal speed between neurons.
Oligodendrocytes are the cells primarily responsible for myelination, the process of insulating nerves so they can transmit information rapidly. Brains with FOXG1 often have high numbers of oligodendrocyte precursor cells (OPC) yet delayed myelination.
According to the study, the therapy normalized the number of OPCs while restoring myelination.
The study provides a solid foundation for advancing the gene therapy toward human clinical trials, researchers said.
"We are thrilled by the full rescue of brain structure abnormalities observed in our mouse model through this study. It marks a significant step forward in our research. With these promising results, we are eager to advance this AAV9 compound toward human clinical trials, hopeful that we can extend these breakthroughs to benefit children with FOXG1 syndrome."
More information: Jeon S. et al, The postnatal injection of AAV9-FOXG1 rescues corpus callosum agenesis and other brain deficits in the mouse model of FOXG1 syndrome. Molecular Therapy: Methods & Clinical Development (2024) doi: doi.org/10.1016/j.omtm.2024.101275.