This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
Dallas cancer tech company develops new blood test it says can catch tumors early
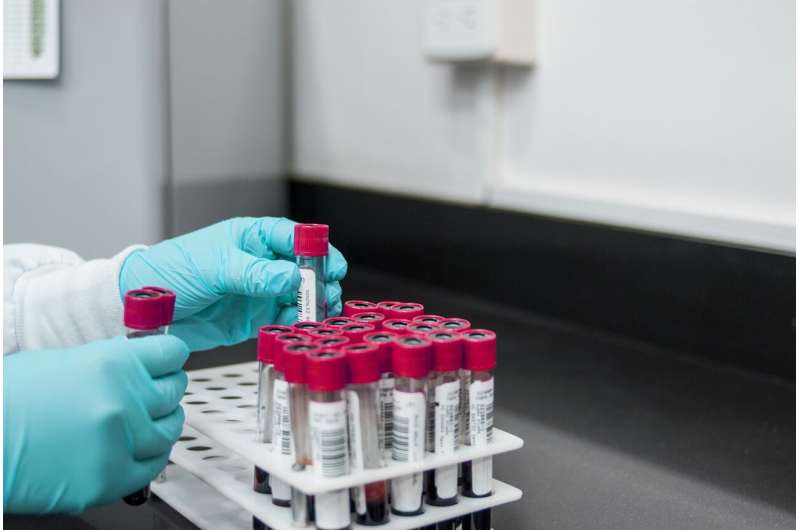
In the fight to beat cancer, scientists have recognized the importance of catching it early.
As a result, a growing market for cancer tests has added to recommendations for cancer-specific screenings at certain ages. A Dallas company has added another product to this market, and says it can detect cancer earlier than ever with a $1,500 blood test. Cancer Check Labs calls the test a "breakthrough" in early detection, testing for more than 200 kinds of cancer with a blood draw.
Cancer is a leading cause of death worldwide, and the second leading cause of death in Texas. Around 40% of men and women will be diagnosed with cancer at some point in their life, according to the National Cancer Institute. In 2023, the American Cancer Society estimated 44,140 new cancer deaths in Texas.
But catching cancer early, before it has grown or spread, makes it easier to treat. The global market for cancer diagnostics was valued at $114.8 billion in 2023 by Dimension Market Research, and is expected to grow at an annual rate of 6.6%.
Within this market, blood tests have risen in popularity as a way to try to detect cancer, but not without trial, error and controversy.
Doctors have concerns over whether or not these tests will improve outcomes for patients. And in 2022, CEO Elizabeth Holmes was found guilty for fraud after her blood testing company, Theranos, misled investors, falsely claiming it could test accurately for cancer and diabetes through a finger stick.
The Food and Drug Administration has not approved the Cancer Check test, although the test is certified through the Clinical Laboratory Improvement Amendments, or CLIA, which regulates clinical lab testing.
The FDA hasn't enforced requirements for most lab developed tests. The agency balances allowing quick development of technology with the need to ensure safety and effectiveness of tests. But in April, the FDA announced it would start regulating lab tests over the course of four years, citing the need to make sure tests work before patients rely on their results.
Despite regulation concerns and with high demand for screens that can find tumors early, companies such as GRAIL and Irving-based Caris Life Sciences are developing tests that screen for multiple cancers at once.
According to a 2021 report by AdvaMed, a medical technology association, Texas ranked sixth in the country for the state with the most revenue from the medical technology industry. The manufacturing of diagnostic tests that look at blood or tissue was the largest sub-sector by global revenue in this industry in 2021, according to Statista.
In this growing market, Dallas-based Cancer Check Labs is adding a technology with a slightly different approach.
How the test works
While many other blood tests look for chemicals, proteins or scraps of DNA shed from tumor cells that could indicate someone has cancer, Cancer Check's technology can extract whole, intact tumor cells from a blood sample. The $1,495 test screens 40 ml of blood, about four vials, and looks for more than 200 types of tumors. The test looks for solid tumors, which grow in organ systems, such as breast cancer, pancreatic cancer and lung cancer.
"The mechanism by which the test operates is very different than everything else in the world," said Sumit Rai, Cancer Check Labs CEO.
The circulating tumor cells, or CTCs, that the company says it can detect based on cells' shared features, are shed from the tumor. They travel through the bloodstream and can indicate that a person may have cancer. Cancer Check says the process it's developed to filter through the blood can extract tumor cells at an "unprecedented scale."
Rai said the Cancer Check test can process a more statistically significant amount of blood than other tests. He said they are also able to mechanically separate tumor cells from the blood sample, processing tests faster and more accurately than other technologies. The company has three patents for its filter system's technology, according to the CEO.
"We've developed a technology that allows us to extract those CTCs from blood and that's extremely different than anything today," Rai said. "We're not using imaging. We are detecting at the cellular level."
In scans that look at images of the body, only tumors that are large enough to show up in the image will be detected. But Rai said that for early detection, finding the presence of cancer in cells themselves is key.
Still, Cancer Check's test is not intended to replace other guidelines and practices for detecting cancer. The American Cancer Society recommends regular cancer screening tests for people without symptoms as young as 21. The CDC recommends screenings as well to try to find breast, cervical, colorectal and lung cancers early.
Challenges in early detection
Dr. Chris Amos, a professor at the Baylor College of Medicine who studies early-stage lung cancer, appreciates the two-step design of the Cancer Check test, where it first identifies if tumor cells are present and then seeks to find which organ the cells came from with a follow-up test from another blood draw.
The test does not screen for blood cancers like leukemia, but Rai said the company is working on developing that technology, too.
Amos said that looking for circulating tumor cells is an emerging and exciting area of research. But there are still many obstacles and challenges in detecting cancer early, and the company has more to do to prove their method works.
Early-stage cancers are smaller and harder to detect. Tests sensitive enough to detect small tumors can also be very expensive, which makes them harder to test on large populations. Cancer Check's price tag of nearly $1,500 is a steep barrier for many customers and for increased testing.
Another challenge with detecting cancer early is knowing whether or not it will actually pose a problem to a person's health, according to the National Cancer Institute. Sometimes a patient's immune system will detect cancer and clear it. Finding cancer early also doesn't always mean it can be effectively treated, researchers at the institute wrote.
The risk of overdiagnosis involves finding cancer that might never have been harmful or could lead to unnecessary treatment. It's not clear yet if screening for certain cancers reduces mortality. Some tests may also detect cancer when it is not in the body, giving a false positive result.
Rai and Dr. Arun Balakumaran, the chairman of the scientific advisory board for Cancer Check Labs, said that because the test functions similar to a tissue biopsy, there isn't much risk of a false positive result with Cancer Check. They place cells on a glass slide, which is analyzed by a pathologist, who determines whether or not there are cancer cells present.
"The false positive with this is zero," Balakumaran said. "And that's another big differentiator with the other tests, which look at parts of the cell, not the whole cell by itself, and can make these erroneous mistakes to come up with false positives."
In May, the company released findings that their test found cancerous cells in a blood sample when the tumor was at stage zero. Stage zero cancer, also called pre-cancer, refers to abnormal cells that might become cancer or spread. The study was co-published with CHRISTUS Cancer Center and the Comprehensive Blood & Cancer Center in California.
"If you can detect that early, you can almost eradicate mortality from cancer and that has profound implications," Rai said. "That is really the key to curing cancer and changing outcomes."
The study is limited, and far from proves that the company's method can detect cancer before it's able to be detected through imaging, Amos of the Baylor College of Medicine wrote in an email. The company's findings were based on three breast cancer patients.
"These are very early proof of concept studies," Amos wrote. "The methods look interesting but scaling up to making publicly available tests and validating the sensitivity and specificity are major factors that need to be accomplished."
Balakumaran said the company is gathering more data and continuing to research their test. The technology is 14 years in the making across different companies, Rai said, and it cost around $80 million to develop the filter technology.
Taking the test
Customers can buy the test online. The company sends a phlebotomist to customers, who use a kit sent from the company to draw the blood samples, which are shipped to the lab in Dallas. Currently, Cancer Check says they can send results back in three to five days, and expects to stay in this range even with more demand for the test.
"We're not using any kind of magic," Rai said. "We don't need weeks and weeks of analysis and processing. We literally just want to look at the tissue. We don't want to guess."
Rai said the company has seen enormous demand for the product, which hit the market this year. The test is also working with dozens of medical practices to supply their patients with the test. Rai said medical practices can trust the board-certified pathology report that comes from the test's analysis.
The company raised the price of the test by around $200 to nearly $1,500 this summer because of more demand from customers and other businesses, Rai said. Cancer Check does not work with insurance companies, but the test is eligible for Health Savings Account and Flexible Spending Account pre-tax benefits.
A passion for cancer research
Rai's endeavors in cancer research are personal. His sister and only sibling died of cancer 12 years ago, and the former Silicon Valley venture capitalist has since dedicated much of his time and investments to cancer research and related initiatives. He said his company is largely self-funded through friends and family.
"I didn't decide to go from venture capital and tech and mobile media into cancer because I saw some huge opportunity to go build a giant company and make money," Rai said. "It was really driven by passion."
Blood tests are an emerging area of research in cancer diagnostics. While many companies have jumped on the possibilities for early diagnosis, doctors say more testing is needed to fully understand their impact in cancer treatment. But for customers who can afford the test, Cancer Check Labs is offering a new technology to try and spot tumors early.
"No one, till today, has been able to detect circulating tumor cells with such high precision," Balakumaran said. "There's not been this nice filtration technique that anyone has."
2024 The Dallas Morning News. Distributed by Tribune Content Agency, LLC.