This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Aceneuramic acid is the first approved drug for GNE myopathy treatment
In order to treat an underserved population of patients with a rare condition whose muscles gradually become weaker until they can no longer walk, a team of researchers across Japan have completed a clinical study to confirm the safety of long-term administration of a therapeutic drug.
Distal myopathy with rimmed vacuoles, or GNE myopathy, is a very rare disease in which muscle atrophy and degeneration occurs in the distal limbs (such as fingers and ankles). Symptoms typically begin from the teens to the early 30s. It gradually leads to a profound loss of motor control. This can greatly affect one's quality of life as patients slowly lose muscle strength, and there are no approved treatments available.
"Despite there being a demand from patients, developing a treatment to slow down symptom progression has been difficult due to the rarity of the disease," remarks Masashi Aoki, a professor from Tohoku University, "For example, there are approximately 400 people with GNE myopathy in all of Japan."
A treatment for a population this size is considered an "ultra orphan drug"—because it is not profitable for pharmaceutical companies to develop treatment for such a small group. As a result, these patients are "orphaned" and left without any help.
Despite these hurdles, a team of researchers stepped in to develop a treatment. Patients with GNE myopathy have reduced functioning of an enzyme that produces sialic acid, so patients were given a drug containing aceneuramic acid (a type of sialic acid) to supplement this deficit.
Researchers conducted investigator-initiated phase I and phase II/III studies, and an efficacy confirmation study sponsored by Nobelpharma Co., Ltd. These studies demonstrated the treatment effects of an ultra orphan drug, "Aceneuramic Acid (Acenobel) Extended Release Tablets 500mg" for GNE myopathy.
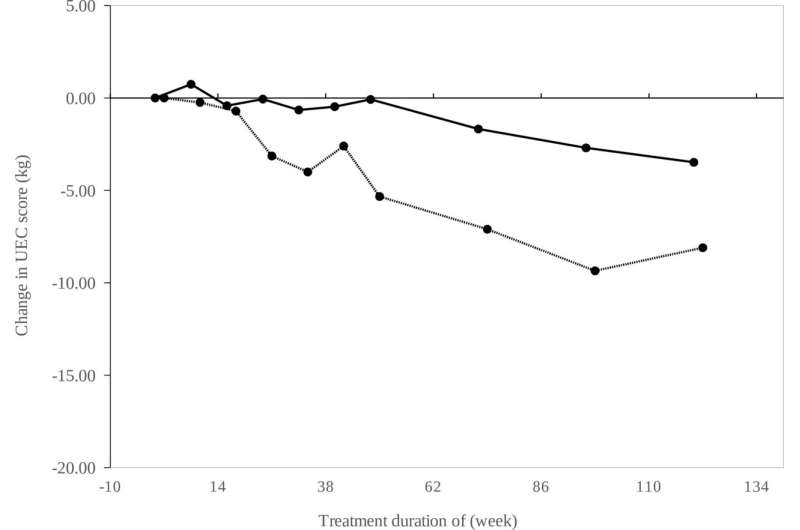
In the current study, 19 patients with GNE myopathy completed a 72-week treatment plan without major adverse effects. These patients originally participated in a 48-week double-blind treatment study to compare the drug to a placebo, and treatment was extended to 72 weeks for this trial.
The safety and efficacy of the treatment was confirmed in the current study, published in the Journal of Neurology, Neurosurgery, and Psychiatry. This led to Nobelpharma Co., Ltd. obtaining official manufacturing and marketing approval from the Ministry of Health, Labor and Welfare in Japan (March 2024). This approval is great news for patients, who finally have a safe, viable treatment option.
The research team plans to continue monitoring the efficacy of the treatment over even longer periods of time.
More information: Naoki Suzuki et al, Safety and efficacy of aceneuramic acid in GNE myopathy: open-label extension study, Journal of Neurology, Neurosurgery & Psychiatry (2024). DOI: 10.1136/jnnp-2024-333853