This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Examining Alzheimer's disease drug impact on tissue samples from people with Down syndrome: Study raises safety concerns
People with Down syndrome are likely to develop Alzheimer's disease at a young age, with autopsy studies showing that by age 40 years, the brains of individuals with Down syndrome have amyloid plaques. Yet people with Down syndrome have been excluded from or underrepresented in clinical trials of new therapies for treating AD. Lecanemab, which has been shown to target and remove beta-amyloid plaques, has been approved by the U.S. Food and Drug Administration to treat AD early in the disease's progression.
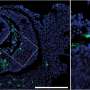
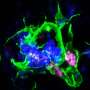
A new study led by investigators at Brigham and Women's Hospital and University of California, Irvine tested lecanemab to see if it could bind to amyloid plaques in tissue samples from people with Down syndrome, finding that it effectively targeted amyloid in all 15 samples. However, the drug also bound to brain blood vessels, which raises safety concerns. Results are published in JAMA Neurology.
"Our study is highly clinically relevant, as we focus on the usage of a recently approved disease modifying therapy for Alzheimer's disease, lecanemab, in people with Down syndrome," said co-corresponding author Lei Liu, MD, Ph.D., of the Department of Neurology at Brigham and Women's Hospital.
"Our findings underscore the exciting promise of anti-amyloid drugs for helping people with Down syndrome, but also the need for careful consideration of safety, especially the risk of hemorrhagic complications," said co-corresponding author Elizabeth Head, Ph.D., of the Department of Pathology and Laboratory Medicine at University California, Irvine.
The research team evaluated brain tissue samples from 15 people with Down syndrome who were between the ages of 43 and 68 years. The study was limited in its sample size and age range—in the future, the researchers hope to expand the study to include samples from younger brain donors to determine if age may be a factor in the drug binding to blood vessels.
The team also plans to evaluate the drug's binding profile in people with late-onset AD to see if it follows a similar pattern. The research team expresses its gratitude to the people with Down syndrome for their gift of brain donation.
More information: Lei Liu et al, Lecanemab and Vascular-Amyloid Deposition in Brains of People With Down Syndrome, JAMA Neurology (2024). DOI: 10.1001/jamaneurol.2024.2579