This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves Leqselvi for severe alopecia

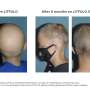
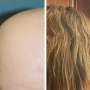
The U.S. Food and Drug Administration has approved Leqselvi (deuruxolitinib) tablets for the treatment of adults with severe alopecia areata.
Leqselvi (8 mg) is a twice-daily oral selective inhibitor of the Janus kinases JAK1 and JAK2. In trials, the three most common adverse events were headache, acne, and nasopharyngitis.
The approval was based on data from the THRIVE-AA1 and THRIVE-AA2 randomized, double-blind, placebo-controlled phase 3 clinical trials, which enrolled a total of 1,220 patients with alopecia areata who had at least 50 percent scalp hair loss for more than six months. Over 24 weeks, more than 30 percent of patients taking Leqselvi experienced ≥80 percent scalp hair coverage (Severity of Alopecia Tool [SALT] ≤20). Up to one-quarter of patients had almost all of their scalp hair back at 24 weeks (≥90 percent coverage). The number of Leqselvi-treated patients achieving a SALT score ≤20 did not plateau through 24 weeks.
"For many people with severe alopecia areata, early intervention with effective treatment is critical," Natasha Mesinkovska, M.D., Ph.D., from the University of California, Irvine, and an investigator in the Leqselvi clinical development program, said in a statement. "An oral JAK that delivers proven results will be impactful for the alopecia areata community."
Approval of Leqselvi was granted to Sun Pharma.
More information: www.multivu.com/players/uk/928 … ere-alopecia-areata/
Copyright © 2024 HealthDay. All rights reserved.