This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How insulin, zinc and pH can block harmful protein clumps linked to type 2 diabetes
An estimated 462 million people around the world suffer from type 2 diabetes, a chronic disease in which the body has problems using sugar as a fuel, leading to a buildup of sugar in the blood and chronic health issues.
New research led by Ayyalusamy Ramamoorthy, a professor at the FAMU-FSU College of Engineering and the Florida State University-headquartered National High Magnetic Field Laboratory, shows how zinc, pH levels and insulin work together to inhibit the buildup of protein clumps that contribute to this disease. The work, which points toward promising avenues for innovative treatments, was published in Communications Biology.
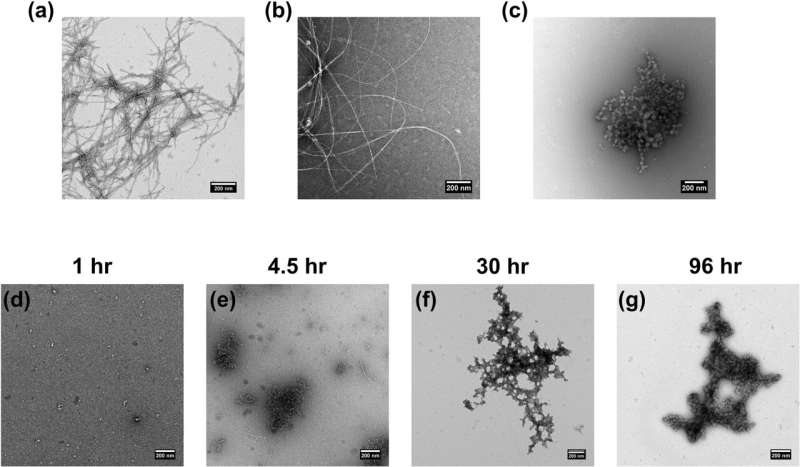
The research focuses on the intricate dance between insulin and the hormone amylin, or human islet amyloid polypeptide (hiAPP). Amylin is a naturally occurring peptide hormone that plays a role in regulating glycemia and energy balance. But human amylin can form amyloid fibers, which can destroy insulin-producing cells in the pancreas.
"At the core of our research we want to understand the complex effects of insulin on amylin's aggregation and its resultant toxicity," said Ramamoorthy, who directed the study. "These are factors that are critical to understanding type 2 diabetes pathophysiology."
What sets this study apart is its innovative angle on enhancing insulin's protective capabilities against IAPP's harmful effects. As the research progresses, new treatments for the millions of people grappling with type 2 diabetes move closer.
"Amylin is produced in the pancreas alongside insulin and has a tendency to clump into aggregates called amyloid," said Sam McCalpin, a post-doctoral researcher in the Ramamoorthy lab at the National High Magnetic Field Laboratory. "They're like the plaques that form in the brain with Alzheimer's or Parkinson's disease."
Researchers are interested in developing drugs to break them up or stop them from forming. For individuals with type 2 diabetes, amylin tends to cluster into harmful amyloid plaques, devastating the islet cells responsible for hormone production. However, insulin emerges as a potential hero, showing capabilities to hinder amylin's aggregation.
This study unravels the nuances of their interaction, alongside the roles of zinc and pH levels, bringing scientists closer to decoding the cellular intricacies of diabetes.
"There is evidence that insulin can help, but it is not potent enough to directly affect type 2 diabetes," McCalpin said. "So, we want to use insulin as a model to engineer more effective treatments in the future."
The results promise not just groundbreaking insights into this biomedical mystery, but also practical solutions. The research will help drug development aimed at neutralizing amylin's toxicity, Ramamoorthy said. This could potentially revolutionize treatment approaches, offering hope to those battling this pervasive illness.
Co-authors on this research were Bernd Reif from the Technical University of Munich, Madalena Ivanova from the University of Michigan and Lucie Khemtemourian from the University of Bordeaux.
More information: Samuel D. McCalpin et al, Zinc and pH modulate the ability of insulin to inhibit aggregation of islet amyloid polypeptide, Communications Biology (2024). DOI: 10.1038/s42003-024-06388-y