This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Multipurpose vaccine shows new promise in the presence of pre-existing immunity
As researchers estimate that nearly all Americans have been exposed to SARS-CoV-2, the virus that causes COVID-19, whether through infection or vaccination, we are no longer "immunologically naive"—in other words, our immune systems are familiar with certain variants of SARS-CoV-2. This familiarity can pose a problem for vaccine development.
In immunology, the phenomenon of "original antigenic sin" or OAS is a colloquial term for the body's first encounter with a virus, which can forever "bias" the immune response to produce antibodies tailored to the initial strain in an exposure, regardless of subsequent infections with other strains or vaccine boosters designed for different viral variants.
Viruses like SARS-CoV-2, including the virus that caused the original SARS epidemic in 2002, belong to a family called SARS-like betacoronaviruses, or sarbecoviruses. Sarbecoviruses have already caused two global health crises in the last 20 years, suggesting a potential for a new sarbecovirus to cause yet another epidemic or pandemic and underscoring a need for broad-based protection from viruses in this family. But does OAS pose a problem for future vaccines designed to broadly protect against sarbecoviruses and SARS-CoV-2 variants of concern?
This question is the subject of a new study conducted in the Caltech laboratory of Pamela Björkman, the David Baltimore Professor of Biology and Biological Engineering and a Merkin Institute Professor.
The study, titled "Mosaic sarbecovirus nanoparticles elicit cross-reactive responses in pre-vaccinated animals," is published in Cell. The work was performed in collaboration with researchers at Rockefeller University in New York and the University of Washington.
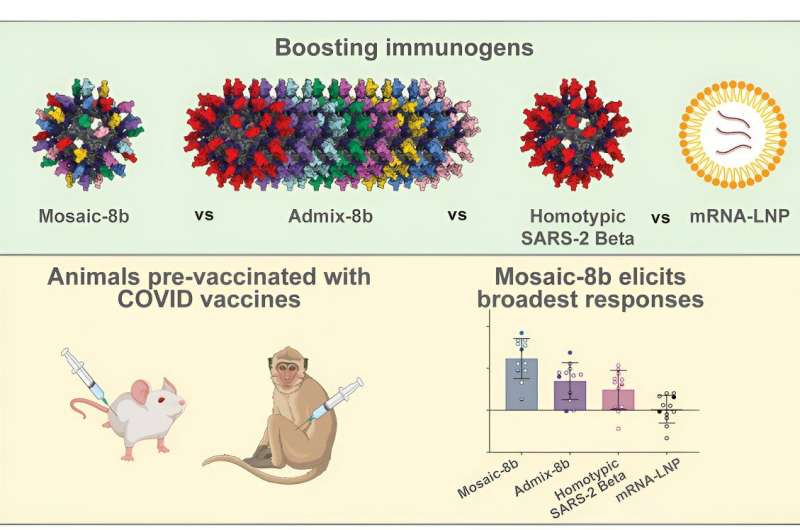
Researchers in the Björkman laboratory have been developing and testing a new vaccine candidate called mosaic-8 that has shown potential to protect against multiple different types of sarbecoviruses, including SARS-CoV-2 and its variants.
In animal models, mosaic-8 induces the production of cross-reactive antibodies, which are able to neutralize multiple sarbecoviruses and SARS-CoV-2 variants.
As mosaic-8 is being prepared for initial human clinical trials, scientists wanted to understand if OAS would influence the composition of elicited antibodies—in other words, how would mosaic-8 influence antibody production in individuals who have already mounted immune responses to SARS-CoV-2, either through infection, immunization, or both? Would the mosaic-8 vaccine produce antibodies that are biased toward SARS-CoV-2, as OAS might suggest, or would it still elicit broad, cross-reactive antibodies against multiple sarbecoviruses and variants?
In the new study, described in a paper appearing in the journal Cell on August 27, examines animal models that had received COVID-19 vaccinations and subsequently built up an immune response. In an encouraging step for the vaccine candidate's development, the team found that mosaic-8 elicits broadly protective cross-reactive antibodies in both previously exposed and immunologically naïve animals.
How mosaic-8 works
When you receive a vaccine for a certain pathogen, your body evolves different antibodies to attack that pathogen and creates B cells that store a memory of how to create those antibodies. Like a lock and key, each antibody protein is specially shaped to attack a specific pathogen. There are trillions of different B cells in your body, each encoding the ability to generate antibodies for specific viruses and other microbes.
After receiving a vaccine against SARS-CoV-2, the body makes many different antibodies that target different regions of the virus. Some of these regions, known as conserved regions, are identical between variants. Antibodies that target conserved regions are more likely to protect against multiple different variants and similar viruses.
The mosaic-8 vaccine is designed to elicit antibodies against conserved features of sarbecoviruses. The vaccine contains pieces of eight different sarbecoviruses. These pieces are regions of the viruses' spike protein called receptor-binding domains (RBDs). The spike protein and its RBDs are crucial for the virus to infect a cell, so the mosaic-8 vaccine is designed to induce the production of cross-reactive antibodies that target conserved regions of RBDs and thus protect against multiple sarbecoviruses and SARS-CoV-2 variants of concern.
Mosaic-8 produces broadly protective antibodies regardless of prior exposure or vaccination
In the study, the team used mouse and nonhuman primate models to determine what kinds of antibodies were elicited by mosaic-8 following previous vaccination with three different COVID-19 vaccines.
The researchers first immunized animals with mRNA vaccines similar to the Pfizer and Moderna vaccines or with the ChAdOx1 vaccine from AstraZeneca, vaccines that are widely available to humans, in order to mimic the situation with humans who are not immunologically naive with respect to SARS-CoV-2. After antibodies were elicited by a vaccine, the animals were then immunized with the team's mosaic-8 vaccine containing RBDs from SARS-CoV-2 and seven related sarbecoviruses.
The researchers then examined antibodies present in the animals' serum. They found that the mosaic-8 vaccine boosted antibodies that target conserved regions of the RBDs of animal sarbecovirus and SARS-CoV-2 variants, and also drove the creation of new antibodies that targeted regions specific to some of the seven animal sarbecovirus RBDs.
"Current COVID-19 vaccines are not specifically designed to generate a broad antibody response that could offer better protection against variants or related viruses," says postdoctoral scholar Alexander Cohen, a co-first author of the new study.
"We developed a vaccine to give broad protection against a spectrum of sarbecoviruses in order to prevent the next pandemic. Fortunately, our new results show that this vaccine functions as designed to elicit broadly protective antibody responses in both previously exposed and immunologically naive animals."
After infection or vaccination, humans produce antibodies that target conserved regions on sarbecoviruses, but those are not the dominant type of antibodies made by the body, says senior research scientist Jennifer Keeffe, co-first author of the study.
"The goal is for our mosaic-8 vaccine to preferentially boost those kinds of antibodies so that the body makes more of them, providing broader protection. We saw these cross-reactive antibodies being produced in our earlier studies in immunologically naive animals, and we have now verified that broadly cross-reactive antibodies are also produced in pre-vaccinated animals. We look forward to confirming this result in our future clinical trials," says Keeffe.
Phase 1 clinical trials to test the mosaic-8 vaccine in humans are scheduled to begin in 2025.
More information: Alexander A. Cohen et al, Mosaic sarbecovirus nanoparticles elicit cross-reactive responses in pre-vaccinated animals, Cell (2024). DOI: 10.1016/j.cell.2024.07.052