This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New safety checklist aims to prevent side effects in patients with arthritis
Inappropriate prescription of an antirheumatic drug for an unsuitable patient can lead to severe side effects such as intestinal perforations, blood clots, heart failure, or liver damage.
To address this issue, researchers from the Department of Biomedicine at Aarhus University and the Rheumatology Department at the University Clinic for Innovative Patient Pathways in Silkeborg have developed a comprehensive safety checklist for the newer medications used in treating rheumatic diseases.
"With the increasing number of medications on the market, it becomes more challenging for health care professionals to make prescriptions without risking serious side effects," explains Associate Professor Tue Wenzel Kragstrup, one of the researchers behind the study and article recently published in the journal Drug Safety.
The article presents the checklist designed to help prevent patients from receiving medications they cannot tolerate.
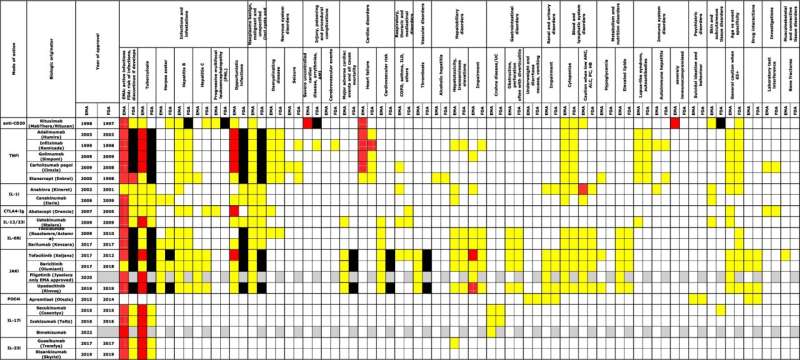
"With over 20 antirheumatic drugs, each with up to 10 specific contraindications or precautions, there is an urgent need for advanced support tools to assist doctors and pharmacists in navigating the medical landscape," adds Tue Kragstrup.
The checklist enables doctors to quickly determine if a patient has comorbidities that limit the use of certain medications.
The principles can be applied across other disease areas
This checklist is the first of its kind, created by analyzing all available patient information leaflets and international treatment guidelines in Europe and the U.S. It is based on data from both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) in the U.S..
Primarily intended for doctors prescribing medications to patients with rheumatic diseases, the study is also relevant to other doctors and patients as it highlights the risks associated with medical treatments and the need for tools to improve prescription safety in general. Dr. Lykke Skaarup, one of the researchers behind the data extraction in the study, explains.
"The principles behind the checklist can be applied across other disease areas because we have documented a method to systematically identify contraindications and precautions for a group of medications," she says.
For example, similar checklists could be created for antihypertensive drugs, migraine medications, or cholesterol-lowering drugs using the same approach.
The study has made a wide range of medication information clear, accessible, and user-friendly, enabling doctors to make more informed decisions. Consequently, they can reduce the risk of side effects and enhance patient safety for those treated for inflammatory rheumatic diseases, both now and in the future.
Tue Wenzel Kragstrup notes that the results align with previous studies highlighting the need for better medication safety.
"We hope our work can contribute to safer and more effective treatment of rheumatic diseases," he says.
The checklist will, of course, need to be continuously updated with new research and the latest reported side effects. The researchers are already working on implementing AI-driven support tools to handle much of this task.
More information: Lykke Skaarup et al, A Systematic Overview of Contraindications and Special Warnings for Biologic and Targeted Synthetic Disease Modifying Antirheumatic Drugs: Establishing a Framework to Create a "Safety Checklist", Drug Safety (2024). DOI: 10.1007/s40264-024-01461-1