This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Blood-based assay shows promise for detecting IDH1.R132H-mutant gliomas
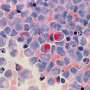
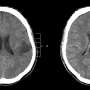
Glioma represents the most common central nervous system cancer in adults. The current classification scheme uses molecular alterations, particularly IDH1.R132H, to stratify lesions into distinct prognostic groups.
Currently, neuroimaging followed by tissue biopsy testing (surgical biopsy and/or resection) is the gold standard for diagnosing IDHI mutant gliomas.
However, this process poses procedural risks and may not fully reflect the diverse and evolving tumor landscape. Also, preoperative knowledge of the IDH mutation status can influence operative procedural planning.
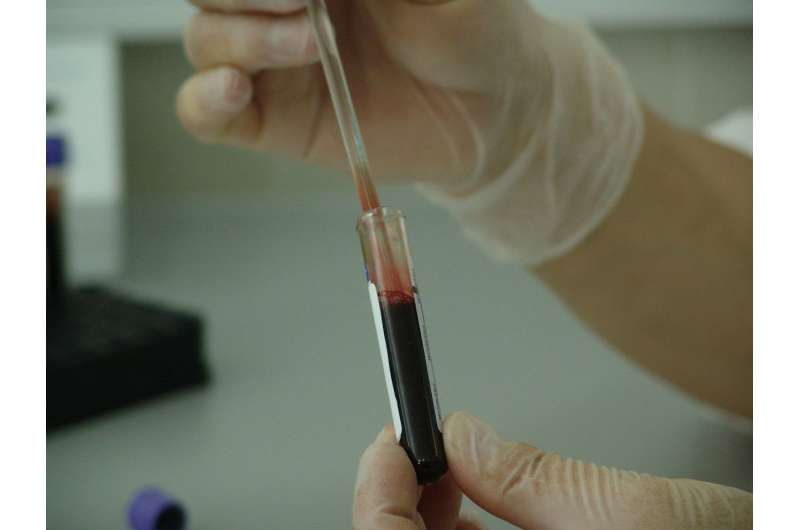
In a study published in Nature Communications, researchers introduce a blood-based test, mt-IDHIdx, that allows for the minimally invasive detection of tumor-derived extracellular RNA using only 2ml of blood.
The researchers performed validation testing across the study population (n=133), which included 80 patients with IDH1.R132H glioma, 44 patients with IDH1 wild-type gliomas and nine healthy controls.
The results from plasma testing demonstrate an overall sensitivity of 75.0% (95% CI: 64.1%–84.0%), specificity 88.7% (95% CI: 77.0%–95.7%), positive predictive value 90.9%, and negative predictive value 70.1% compared to the tissue gold standard.
In addition to fundamental diagnostic applications, the study also highlights the utility of the platform for blood-based monitoring and surveillance.
Finally, the optimized workflow enables rapid and efficient completion of both tumor tissue and plasma testing in under four hours from the time of sampling.
Detection of the IDH1 mutation in blood means that these tumors can be diagnosed without a biopsy, and can be monitored overtime to track disease progression, response to treatment or recurrence using a simple blood sample.
Importantly, the FDA approved a drug (vorasidenib) for this mutation in glioma in August 2024—this means that the test, once approved, can be used to stratify these patients for this treatment and can monitor disease course.
More information: Syeda Maheen Batool et al, Clinical utility of a blood based assay for the detection of IDH1.R132H-mutant gliomas, Nature Communications (2024). DOI: 10.1038/s41467-024-51332-7