This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
FDA approves drug targeting brain cancer gene mutation
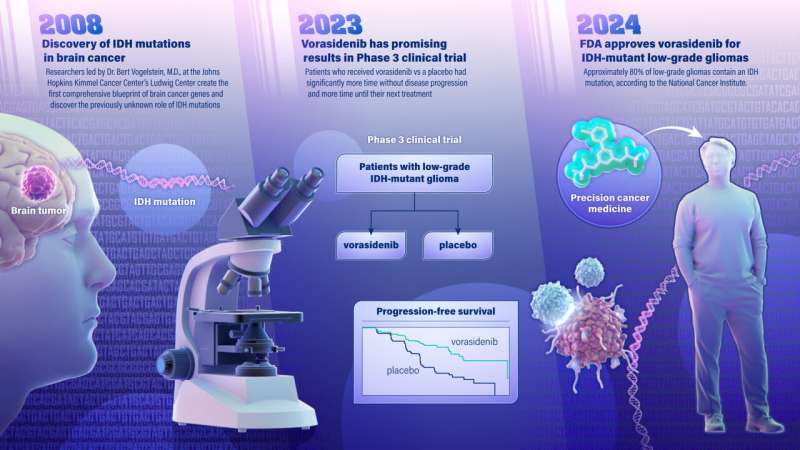
A new drug for treatment of a type of brain cancer, called IDH-mutant low-grade glioma, was approved Aug. 6 by the U.S. Food and Drug Administration (FDA). The promising new drug stems from a 2008 genetic discovery made at the Johns Hopkins Kimmel Cancer Center.
The drug, called vorasidenib, is a targeted cancer therapy that works by inhibiting the activity of a mutated gene called IDH, slowing the growth of the cancer. The gene was identified by Bert Vogelstein, M.D., and team at the Johns Hopkins Kimmel Cancer Center's Ludwig Center in 2008 when they became the first to map the genetic blueprint for brain cancer. The blueprint was considered the most comprehensive genetic analysis for any tumor type, evaluating all known protein-encoding genes in brain cancer.
The researchers found that the IDH gene—which had never been suspected to be involved in any tumor type—was frequently mutated in a subset of brain cancers.
"IDH is the poster child for cancer genome sequencing, and it illustrates the importance of basic research," says Vogelstein, the Clayton Professor of Oncology, Howard Hughes Medical Institute investigator, and co-director of the Ludwig Center.
"The history of medicine shows that when a disease is understood, it eventually becomes manageable. It may not be immediately evident, but in time, as in this case, such discoveries result in better treatment for patients."
Vogelstein and team's genetic discoveries ushered in what is known as precision, or individualized, cancer medicine in which therapies are targeted to the unique genetic makeup of each patient's cancer.
In June 2023, findings from a multicenter, phase 3 clinical trial of vorasidenib in 331 patients with IDH-mutant low grade glioma was published in the New England Journal of Medicine and concluded that patients with grade 2 IDH-mutant glioma who received the drug had significantly improved progression-free survival and that the therapy delayed the time to the next intervention compared to patients who received a placebo. The trial was sponsored by the international pharmaceutical company Servier, who is bringing vorasidenib to market.
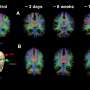
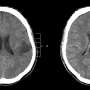
In addition to this newly FDA-approved drug, the IDH gene discovery led to a new classification of gliomas, differentiating cancers with an IDH mutation that have an overall better outcome and response to treatment of very aggressive gliomas without an IDH mutation, including glioblastoma, the most common primary brain cancer in adults. It has also paved the way for additional studies into other types of brain cancer.
Approximately 80% of low-grade gliomas contain an IDH mutation, according to the National Cancer Institute. They include IDH-mutant astrocytoma and oligodendroglioma, and they occur most commonly in younger adults. Low grade gliomas tend to be slower growing and are associated with longer survival than aggressive, high-grade gliomas.
Treatments include surgery to remove as much of the tumor as possible, followed by radiation and chemotherapy to attack remaining cancer cells. In some patients, the addition of the IDH inhibitor could delay the need for radiation therapy and chemotherapy.
"The possibility of delaying radiation therapy and chemotherapy with this drug could be beneficial to select patients with slow growing IDH-mutant gliomas," says Matthias Holdhoff, M.D., Ph.D., co-director of the Johns Hopkins Kimmel Cancer Center brain tumor program and a co-investigator on the 2023 clinical trial.
"I believe we are looking at a new standard of care option for these types of tumors."