This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New drug could be breakthrough for brain tumor that strikes young people, says expert
A top UVA Health cancer expert is highlighting how a new drug could transform how doctors treat a brain tumor that typically strikes younger people.
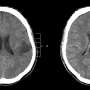
David Schiff, MD, the co-director of UVA Cancer Center's Neuro-Oncology Center, has authored an editorial in the New England Journal of Medicine describing the potential significance of the drug vorasidenib for patients with tumors known as "grade 2 IDH-mutant gliomas." The drug, when tested in the INDIGO clinical trial, was found to slow tumor growth significantly and extended the average time until the tumor started growing from 11.1 months to more than 27 months.
If the drug receives approval from the federal Food and Drug Administration, it would become the first targeted therapy for low-grade gliomas. But Schiff notes that there are also other recent advances that are improving our understanding of such gliomas.
"It used to be that we thought of all gliomas as being on a spectrum," Schiff said. "We now understand that those with the IDH gene mutation have a markedly different biology, outcome and, as this study shows, vulnerabilities that new therapies can exploit."
About IDH-mutant gliomas
Approximately 2,500 Americans with a median age of only 40 are diagnosed with grade 2 IDH-mutant gliomas each year. The tumors often affect the patients' ability to think and hold a job, as well as interfere with other aspects of daily life. Eventually the tumors become resistant to treatment options and typically prove fatal.
Because of the limited treatment options available, doctors usually take a "watch and wait" approach to managing the gliomas, holding off on treatment until after the tumor progresses. But vorasidenib could change that, Schiff notes. The drug could offer the first early treatment for the cancer, giving patients an important new option that could extend their lives.
In the INDIGO trial, more than 300 patients were randomized to receive vorasidenib or a harmless placebo. Neither the patients nor their doctors knew which the patients were receiving. Schiff, in his editorial, describes the results as "striking." Not only did the patients receiving vorasidenib live longer, but they did not need more toxic treatments, such as radiation and chemotherapy, as quickly as the patients receiving placebos.
Schiff was so impressed by the success of the drug that he writes that vorasidenib could "put a nail in the coffin" of the watch-and-wait approach.
"There are still many unanswered questions about how we can best utilize this new medication if and when it receives FDA approval," Schiff said. "Nonetheless, considering that existing standard therapies for these tumors [radiation and chemotherapy] are tough on patients, with short- and long-term side effects, it will be wonderful to have a useful and very well-tolerated treatment option."
Both the results of the INDIGO trial and Schiff's editorial have been published in the New England Journal of Medicine.
More information: David Schiff, Headway against Brain Tumors with Molecular Targeting of IDH-Mutant Gliomas, New England Journal of Medicine (2023). DOI: 10.1056/NEJMe2305639