This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Drug shows promise for treating brain tumors resulting from breast cancer, trial reports
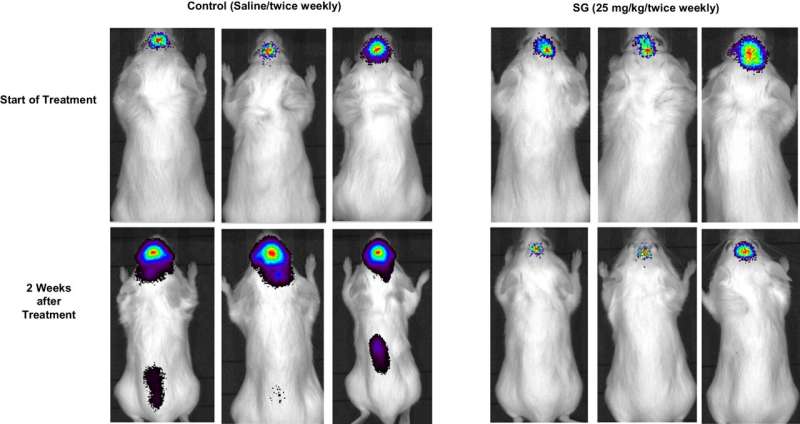
A drug effective in treating breast cancer shows new promise in addressing breast cancer with brain metastases or recurrent glioblastoma, as reported by results of a prospective window-of-opportunity trial at the University of Texas Health Science Center at San Antonio (UT Health San Antonio).
The window trial, in which patients agreed to receive a novel treatment before undergoing surgery, found that the drug sacituzumab govitecan was well-tolerated and showed signs of effectiveness for those whose breast cancer had progressed to brain tumors.
About half of all women with the aggressive and advanced triple-negative form of breast cancer will be diagnosed with brain metastases, and the prognosis is poor, with a median overall survival of just more than seven months.
"We knew that the drug has been effective in the treatment of breast cancer, but its usefulness in treatment of resulting brain tumors has been unclear," said Andrew J. Brenner, MD, Ph.D., professor and chair of neuro-oncology research with Mays Cancer Center at UT Health San Antonio. "Our trial, however, revealed that it could achieve concentrations of inhibitors inside the tumors sufficient to benefit patients, and with minimal side effects, which is very promising for new therapy."
Brenner, who also is clinical investigator for the Institute for Drug Development at UT Health San Antonio and co-leader of its Experimental and Development Therapeutics Program, is lead author of the trial's study published Aug. 7 in Nature Communications, titled "Sacituzumab Govitecan in patients with breast cancer brain metastases and recurrent glioblastoma: a phase 0 window-of-opportunity trial."
Addressing an unmet need
Brain tumors originating from breast cancer are frequent, and treatment for these tumors typically involves surgery, radiotherapy and systemic therapies, though these measures are often unsuccessful.
Similarly, glioblastoma multiforme is the most common primary brain malignancy in adults, representing half of such tumors. It is also the most aggressive primary brain tumor, with a median survival of only 20.9 months despite surgery, radiotherapy, chemotherapy and tumor-treating regimens.
For those reasons, there has been an unmet need to address breast cancer with brain metastasis and recurrent glioblastoma multiforme, and treatment of both primary and secondary brain tumors is limited by many factors.
Sacituzumab govitecan (SG) is a known antibody-drug conjugate, meaning it's a biopharmaceutical drug designed as a targeted therapy for treating cancer. Unlike chemotherapy, it is intended to target and kill tumor cells while sparing healthy cells. It has been demonstrated to be effective in patients with TROP-2, which is a membrane protein found in many tumors.
The trial at UT Health San Antonio enrolled 25 patients aged 18 or older who had been diagnosed with breast cancer with brain metastases or recurrent glioblastoma. Each received a single intravenous dose of the drug one day before tumor-tissue removal and continuing on days one and eight of 21-day cycles following recovery. The timeframes were eight months for patients with breast cancer with brain metastases and two months for those with recurrent glioblastoma.
The researchers discovered significant penetration of the topoisomerase inhibitor SN-38 inside the tumors that was delivered by the drug to fight their development, and without unexpected adverse effects on the patients.
"SG could achieve intra-tumoral concentrations of SN-38 sufficient for therapeutic benefit in patients with brain metastases from breast cancer and recurrent glioblastoma," the researchers concluded. "The drug was well-tolerated in this population with promising clinical signals of efficacy."
They believe the data supports ongoing investigation in a phase 2 clinical trial of this drug in recurrent glioblastoma.
"We currently are in the interim analysis stage of that phase 2 trial, which enrolled 20 patients at UT Health San Antonio, Cleveland Clinic and Texas Oncology in Austin," said William Kelly, MD, assistant professor of hematology and oncology at Mays Cancer Center, and principal investigator of the phase 2 trial. "We expect it will shed further light on the possible effectiveness of SG in treating glioblastoma."
Other authors of the window-trial study also are with Mays Cancer Center, as well as the departments of neurosurgery, obstetrics and gynecology, and pathology and laboratory medicine at UT Health San Antonio; Department of Population Health Sciences at Greehey Children's Cancer Research Institute at UT Health San Antonio; START Center for Cancer Care in San Antonio; and the departments of nutritional sciences, oncology and pediatrics with the Dell Medical School at the University of Texas at Austin.
More information: Henriette U. Balinda et al, Sacituzumab Govitecan in patients with breast cancer brain metastases and recurrent glioblastoma: a phase 0 window-of-opportunity trial, Nature Communications (2024). DOI: 10.1038/s41467-024-50558-9