This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Anlotinib plus STUPP: A new hope for glioblastoma patients
Glioblastoma multiforme (GBM) is among the most aggressive forms of brain tumors, with few effective treatment options and a bleak prognosis. The current standard of care (SOC), known as the STUPP regimen, includes surgical resection, radiotherapy, and temozolomide chemotherapy. Despite this rigorous approach, median progression-free survival (PFS) and overall survival (OS) are typically low.
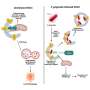
A study published in Cancer Biology & Medicine in March 2024 suggests that adding anlotinib, a multi-kinase inhibitor, to the STUPP regimen could improve outcomes for glioblastoma patients. Given the high vascularity of glioblastomas, the study assessed the effectiveness of combining anlotinib with the STUPP regimen.
The STUPP regimen involves radiation therapy with concurrent and adjuvant temozolomide. Even with this treatment, glioblastoma has a high rate of disease progression within a year.
The trial enrolled 33 patients newly diagnosed with glioblastoma between March 2019 and November 2020. Patients were administered the STUPP regimen in combination with anlotinib at a daily dose of 8 mg, taken continuously for 14 days every 21 days, throughout the duration of their radiotherapy and chemotherapy treatments.
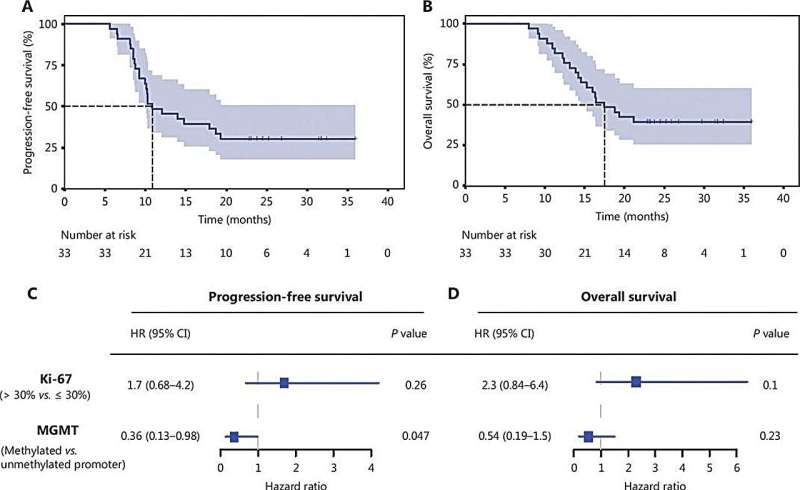
Following the completion of chemotherapy, maintenance therapy with anlotinib as a monotherapy was initiated. The study evaluated the efficacy of this combination in extending PFS and OS, showing promising results with a median PFS of 10.9 months and a median OS of 17.4 months. The 12-month PFS and OS rates were 48.5% and 81.8%, respectively, indicating that adding anlotinib might significantly improve outcomes for glioblastoma patients.
Yuanyuan Chen, a co-author of the study, said, "Anlotinib plus the STUPP regimen has shown promising anti-tumor activity in glioblastoma while maintaining a tolerable toxicity profile. The results could lead to new treatment strategies for this challenging cancer."
These findings could open new doors for glioblastoma treatment, offering hope to patients with this aggressive cancer. Further studies are planned to explore the full potential of this combination therapy and its effects on survival and quality of life.
More information: Shuzhen Lai et al, Efficacy and safety of anlotinib combined with the STUPP regimen in patients with newly diagnosed glioblastoma: a multicenter, single-arm, phase II trial, Cancer Biology & Medicine (2024). DOI: 10.20892/j.issn.2095-3941.2023.0373