This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Mouse study suggests cancer drug could be used to target protein connection that spurs Parkinson's disease
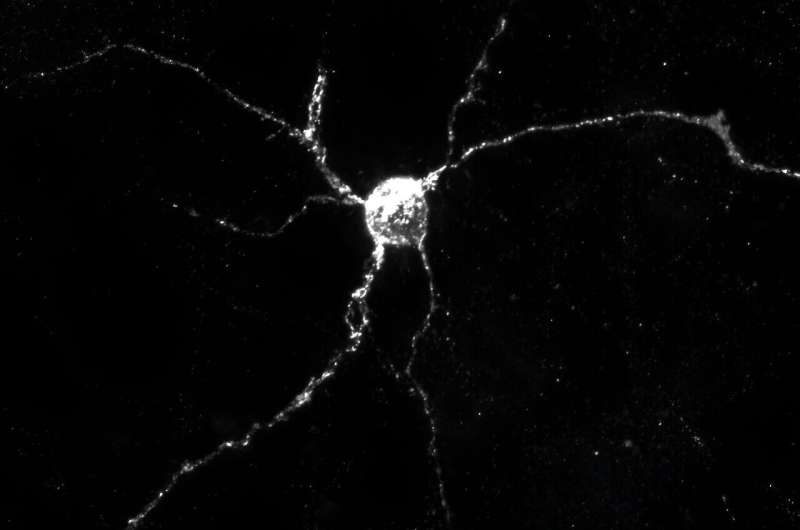
In studies with genetically engineered mice, Johns Hopkins Medicine researchers say they have identified a potentially new biological target involving Aplp1, a cell surface protein that drives the spread of Parkinson's disease-causing alpha-synuclein.
The findings, published May 31 in Nature Communications, reveal how Aplp1 connects with Lag3, another cell surface receptor, in a key part of a process that helps spread harmful alpha-synuclein proteins to brain cells. Those protein buildups are hallmarks of Parkinson's disease.
Notably, the researchers say, Lag3 is already the target of a combination cancer drug approved by the U.S. Food and Drug Administration (FDA) that uses antibodies to "teach" the human immune system what to seek and destroy.
"Now that we know how Aplp1 and Lag3 interact, we have a new way of understanding how alpha-synuclein contributes to the disease progression of Parkinson's disease," says Xiaobo Mao, Ph.D., associate professor of neurology at the Johns Hopkins University School of Medicine and a member of the Institute for Cell Engineering.
"Our findings also suggest that targeting this interaction with drugs could significantly slow the progression of Parkinson's disease and other neurodegenerative diseases."
Mao co-led the research along with Ted Dawson, M.D., Ph.D., Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases at the Johns Hopkins University School of Medicine and director of the Johns Hopkins Institute for Cell Engineering, Valina Dawson, Ph.D. and Hanseok Ko, Ph.D., professors of neurology at the school of medicine and members of the Institute for Cell Engineering.
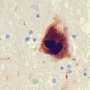
Long-standing studies have shown that by clumping together and forming protein deposits, misfolded alpha-synuclein proteins journey from brain cell to brain cell, killing those responsible for producing a neurotransmitter called dopamine, and causing Parkinson's disease to progress through a type of "programmed" cell death that Johns Hopkins researchers have identified. The process, parthanatos (from the Greek word for "death"), leads to impairments in movement, emotional regulation and thinking.
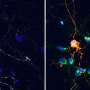
Aplp1's bond with Lag3 on the cell's surface enables healthy brain cells to absorb traveling clumps of alpha-synuclein, leading to cell death, the researchers say.
In mouse studies published in 2016 and 2021, Mao and Dawson's team identified Lag3's role in binding with alpha-synuclein proteins, causing Parkinson's disease to spread. However, those studies indicated that another protein was partially responsible for the cell's absorption of misfolded alpha-synuclein.
"Our work previously demonstrated that Lag3 wasn't the only cell surface protein that helped neurons absorb alpha-synuclein, so we turned to Aplp1 in our most recent experiments," says Valina Dawson.
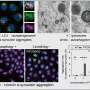
To determine whether Aplp1 indeed contributed to the spread of harmful alpha-synuclein proteins, researchers used a line of genetically engineered mice lacking either Aplp1 or Lag3 or both Aplp1 and Lag3. In mice without Aplp1 and Lag3, cell absorption of the harmful alpha-synuclein protein dropped by 90%.
After injecting mice with the Lag3 antibody, they found that this drug also blocks the interaction of Aplp1 and Lag3, meaning healthy brain cells could no longer absorb disease-causing alpha-synuclein clumps.
The researchers say the Lag3 antibody nivolumab/relatlimab, a drug FDA approved in 2022 for cancer treatment, could play a role in preventing cells from absorbing alpha-synuclein.
"The anti-Lag3 antibody was successful in preventing further spread of alpha-synuclein seeds in the mouse models and exhibited better efficacy than Lag3-depletion because of Aplp1's close association with Lag3," Ted Dawson says.
This research has potential applications in treating other neurodegenerative conditions that have no cures, Mao says. In Alzheimer's disease, which is associated with symptoms of memory loss, mood instability and muscle problems, tau proteins become misfolded and clump together in neurons at high levels, worsening the condition. In Alzheimer's research, Mao says scientists could try to target Lag3—which also binds with the dementia-related tau protein—with the same antibody.
With the success of using the Lag3 antibody in mice, Ted Dawson says the next steps would be to conduct anti-Lag3 antibody trials in mice with Parkinson's disease and Alzheimer's disease. The Johns Hopkins researchers are also looking into how they could prevent unhealthy cells from releasing disease-causing alpha-synuclein in the first place.
More information: Xiaobo Mao et al, Aplp1 interacts with Lag3 to facilitate transmission of pathologic α-synuclein, Nature Communications (2024). DOI: 10.1038/s41467-024-49016-3