This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cell engineering team links cancer drug to potential therapy for Alzheimer's disease
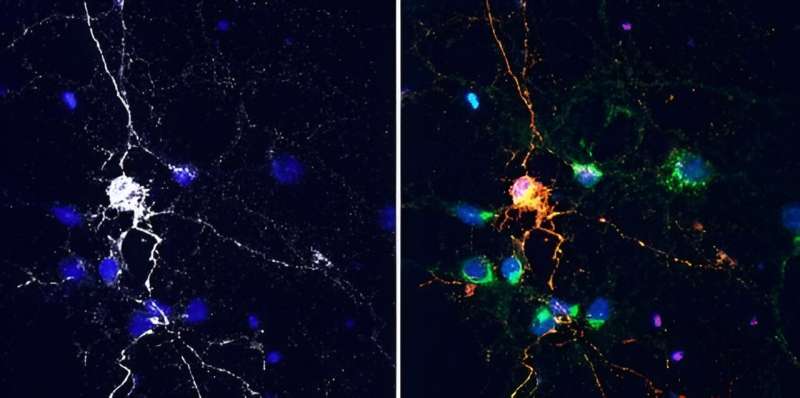
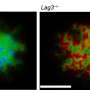
In experiments with lab-grown brain cells and mouse models, scientists at Johns Hopkins Medicine say they have discovered that a cell surface protein called Lag3 is not only a biological target for FDA-approved drugs that stimulate the immune system to attack cancer, but also may be a target for a drug designed to clean up "misfolded" Tau proteins linked to Alzheimer's disease.
Lag3 is found on the surface of many types of cells, including neurons in the brain. Typically, the protein acts to shield cells from attack by the immune system, and it's the target of a recently approved drug for melanoma, designed to make the cancer more vulnerable to immune system attack.
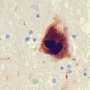
The new findings, published Feb. 7 in Advanced Science, conclude that Lag3 also has an affinity for binding and spreading misfolded or abnormal Tau proteins known to cause Alzheimer's disease.
Tau proteins ordinarily work to stabilize a cell's scaffolding structure of microtubules. In the case of Alzheimer's disease, Tau proteins detach from their scaffolding structure, become misfolded, clump together and accumulate in neurons at abnormal levels. This is a key factor driving progression of Alzheimer's disease, say the scientists.
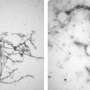
One of the first clues to the Lag3-Tau protein link occurred when the Johns Hopkins scientists, led by Xiaobo Mao, Ph.D., associate professor of neurology at the Johns Hopkins University School of Medicine and member of the Institute for Cell Engineering, found in 2016 and 2021 that Lag3 binds to alpha-synuclein proteins, another type of protein that, when misfolded, causes neurodegeneration common in Parkinson's disease.
In that study, they also found Lag3 does not bind to a benign type of Tau protein that does not cause neurodegeneration. However, they wondered whether Lag3 does bind to the specific type of Tau protein that causes Alzheimer's disease.
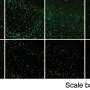
For the current study in lab-grown brain cells, the scientists found that Lag3 did indeed bind to a pathogenic form of Tau protein circulating in spaces near neurons. The cells then "swallowed" the protein.
To find out what happens to neurons without Lag3, the scientists genetically engineered mice to lack Lag3. In these mouse brain cells, fewer tangled Tau proteins were shuttled into the neurons.
Finally, to the mice with active Lag3, the scientists gave antibody drugs aimed at binding to and blocking the protein. They found reduced spreading of Tau proteins in neurons and fewer behavioral deficits, such as more normal sociability, in the animals.
"These findings are particularly promising, and the discoveries in this paper open the door to conducting preclinical studies repurposing FDA-approved Lag3 antibodies for melanoma to treat Alzheimer's disease by interfering with Lag3's ability to propagate toxic Tau proteins," says Ramhari Kumbhar, Ph.D., co-first author and research associate at the Johns Hopkins University School of Medicine.
"Our ultimate aim is to continue studying how a Lag3 antibody drug could be used for Alzheimer's disease," says Mao.
More information: Chan Chen et al, Lymphocyte‐Activation Gene 3 Facilitates Pathological Tau Neuron‐to‐Neuron Transmission, Advanced Science (2024). DOI: 10.1002/advs.202303775