Cancer cells feed on sugar-free diet
(Medical Xpress) -- Cancer cells have been long known to have a “sweet tooth,” using vast amounts of glucose for energy and for building blocks for cell replication.
Now, a study by a team of researchers at Johns Hopkins and elsewhere shows that lymph gland cancer cells called B cells can use glutamine in the absence of glucose for cell replication and survival, particularly under low-oxygen conditions, which are common in tumors.
Writing in the Jan. 4, 2012, edition of Cell Metabolism, Anne Le, M.D., and a team of investigators collaborating with the Johns Hopkins Brain Science Institute, say the finding is critical for developing innovative cancer therapies because it offers “proof of concept” evidence that curbing the growth of B cell cancers can be accomplished by inhibiting a glutamine enzyme called glutaminase.
Le notes that although little is known about glutamine’s role in the growth of B cell cancer, the amino acid circulates in the blood at the highest level among the 20 amino acids that do so.
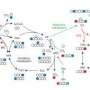
The tricarboxylic acid cycle (TCA or Krebs cycle) is classically regarded as a pathway for glucose oxidation. However, the experiments by Le and the team show that B cells oxidize glutamine when glucose is absent.
The study also found that when oxygen is scarce, there is enhanced conversion of glutamine to glutathione, an important agent for controlling the accumulation of oxygen-containing chemically reactive molecules that cause damage to normal cells.
When the investigators used a glutaminase inhibitor, cancerous growth of B cells was stopped in petri dishes.
“The flexibility of the TCA cycle in using both glutamine and glucose pathways may be important for cancer cells to proliferate and survive, especially under the low-oxygen and nutrient-deprived conditions often encountered in the tumor microenvironment,” says Le.
Now, perhaps, scientists can exploit that survival strategy to stop cancer, according to former Johns Hopkins scientist Chi Dang, M.D., now at the Abramson Cancer Center at the University of Pennsylvania. “A broader and deeper understanding of cancer cell metabolism and cancer cells’ability to reprogram biochemical pathways under metabolic stress can be a rich ground for therapeutic approaches targeting tumor metabolism,” he says.
In addition to Le, an assistant professor in the Department of Pathology at the Johns Hopkins University School of Medicine, other researchers from Johns Hopkins who participated in this study include Sminu Bose, Arvin Gouw, Joseph Barbi, Takashi Tsukamoto, Camilo J. Rojas and Barbara Slusher. The Johns Hopkins Brain Science Institute, where Tsukamoto, Rojas and Slusher are faculty, is pursuing the development of new glutaminase inhibitor drugs.