In drug-approval race, US FDA ahead of Canada, Europe
The U.S. Food and Drug Administration (FDA) generally approves drug therapies faster and earlier than its counterparts in Canada and Europe, according to a new study by Yale School of Medicine researchers. The study counters perceptions that the drug approval process in the United States is especially slow.
Led by second-year medical student Nicholas Downing and senior author Joseph S. Ross, M.D., assistant professor of internal medicine at Yale School of Medicine, the study will be published May 16 online by the New England Journal of Medicine.
Regulatory review represents the final step in the process of bringing new medical technologies from the lab to the bedside. Efficient regulatory review processes may enable patients to get access to promising new therapies sooner, while ensuring drug safety.
"The perception that the FDA is too slow implies that sick patients are waiting unnecessarily for regulators to complete their review of new drug applications," said Downing, who decided to conduct the study because there have been no recent comparisons of the FDA's regulatory review speed with those of regulating agencies in other countries.
Downing, Ross, and colleagues reviewed drug approval decisions of the FDA, the Canadian drug regulator, Health Canada, and the European Medicines Agency (EMA) between 2001 and 2010. They studied each regulator's database of drug approvals to identify novel therapeutics as well as the timing of key regulatory events, allowing regulatory review speed to be calculated. Canada and Europe were chosen as a comparison because they face similar pressures to approve new drugs quickly while ensuring they do not put patients at risk.
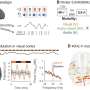
The team found that the median total time to review was 322 days at FDA, 366 days at EMA and 393 days at Health Canada.
"Among the subsample of drugs approved for all three regulators, the FDA's reviews were over three months faster than those of the EMA or Health Canada," said Downing. "The total review time at the FDA was faster than EMA, despite the FDA's far higher proportion of applications requiring multiple regulatory reviews."
Downing added that most new drug therapies were first approved for use in the U.S. "Examining novel drugs approved in multiple markets, we found that 64% of medicines approved in both the U.S. and in Europe were approved for U.S. patients first, and 86% of medicines approved in both the U.S. and Canada were also approved first in the U.S." he said.
More information: New England Journal of Medicine, doi: 10.1056/NEJMoa1200223