Research yields two 'firsts' regarding protein crucial to human cardiac function
Florida State University researchers led by physics doctoral student Campion Loong have achieved significant benchmarks in a study of the human cardiac protein alpha-tropomyosin, which is an essential, molecular-level component that controls the heart's contraction on every beat.
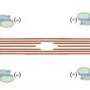
Using an imaging method called atomic force microscopy, Loong achieved two "firsts": the first direct imaging of individual alpha-tropomyosin molecules, which are very small—roughly 40 nanometers long—and the first demonstrated examples of a measure of the human cardiac protein's flexibility. From there, he established a baseline of how flexible a normal version of the protein is supposed to be in a healthy human heart.
"This basic research is important to broadening our understanding of how the human heart functions normally at the molecular level," Loong said. "The flexibility of alpha-tropomyosin dictates how effectively or properly the heart muscle will contract on each beat and has implications for keeping the heart free of cardiovascular disease.
"Before this study, we did not know how flexible this protein was," Loong said. "Using these results, now we can conduct subsequent studies to compare disease-related mutants of this protein to see how much they deviate from normal versions."
Loong served as the lead author of the paper "Persistence Length of Human Cardiac a-Tropomyosin Measured by Single Molecule Direct Probe Microscopy," which was published in the journal PLoS ONE. He conducted the research with physics Professor Huan-Xiang Zhou and biological science Professor P. Bryant Chase, both of Florida State.
When an electrical signal is generated in the heart to make it contract, calcium is released inside each heart muscle cell. The calcium then binds to a protein called troponin, and that triggers the "flexing movement" of alpha-tropomyosin, which allows another protein called myosin—the motor protein—to interact with the troponin/tropomyosin actin filaments. This series of events is what generates the heart's contraction that pumps blood. A subsequent removal of calcium inside each heart cell is what relaxes the heart, which allows the heart to fill with blood to be pumped on the next beat.
"Alpha-tropomyosin is a key element that makes the calcium signal either turn the heart on, making it contract, or turn it off, making it relax," Chase said. "There is an optimal range of flexibility of alpha-tropomyosin for the normal heart to function properly. The molecule can be too stiff or it can be too flexible, either of which could lead to cardiovascular disease. What we ultimately think is that evolution has tuned the mechanical properties of these proteins for optimal function in the heart."